Filling the gap: adding super-resolution to array tomography for correlated ultrastructural and molecular identification of electrical synapses at the C. elegans connectome
- PMID: 27175373
- PMCID: PMC4855082
- DOI: 10.1117/1.NPh.3.4.041802
Filling the gap: adding super-resolution to array tomography for correlated ultrastructural and molecular identification of electrical synapses at the C. elegans connectome
Abstract
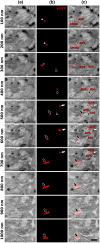
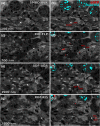
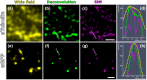
Correlating molecular labeling at the ultrastructural level with high confidence remains challenging. Array tomography (AT) allows for a combination of fluorescence and electron microscopy (EM) to visualize subcellular protein localization on serial EM sections. Here, we describe an application for AT that combines near-native tissue preservation via high-pressure freezing and freeze substitution with super-resolution light microscopy and high-resolution scanning electron microscopy (SEM) analysis on the same section. We established protocols that combine SEM with structured illumination microscopy (SIM) and direct stochastic optical reconstruction microscopy (dSTORM). We devised a method for easy, precise, and unbiased correlation of EM images and super-resolution imaging data using endogenous cellular landmarks and freely available image processing software. We demonstrate that these methods allow us to identify and label gap junctions in Caenorhabditis elegans with precision and confidence, and imaging of even smaller structures is feasible. With the emergence of connectomics, these methods will allow us to fill in the gap-acquiring the correlated ultrastructural and molecular identity of electrical synapses.
Keywords: Caenorhabditis elegans; correlative light and electron microscopy; direct stochastic optical reconstruction microscopy; gap junction; structured illumination microscopy; super-resolution microscopy.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

