PI3K p110β isoform synergizes with JNK in the regulation of glioblastoma cell proliferation and migration through Akt and FAK inhibition
- PMID: 27176481
- PMCID: PMC4866398
- DOI: 10.1186/s13046-016-0356-5
PI3K p110β isoform synergizes with JNK in the regulation of glioblastoma cell proliferation and migration through Akt and FAK inhibition
Abstract
Background: Glioblastoma multiforme is the most aggressive malignant primary brain tumor, characterized by rapid growth and extensive infiltration to neighboring normal brain parenchyma. Both PI3K/Akt and JNK pathways are essential to glioblastoma cell survival, migration and invasion. Due to their hyperactivation in glioblastoma cells, PI3K and JNK are promising targets for glioblastoma treatment.
Methods: To investigate the combination effects of class IA PI3K catalytic isoforms (p110α, p110β and p110δ) and JNK inhibition on tumor cell growth and motility, glioblastoma cells and xenografts in nude mice were treated with isoform-selective PI3K inhibitors in combination with JNK inhibitor.
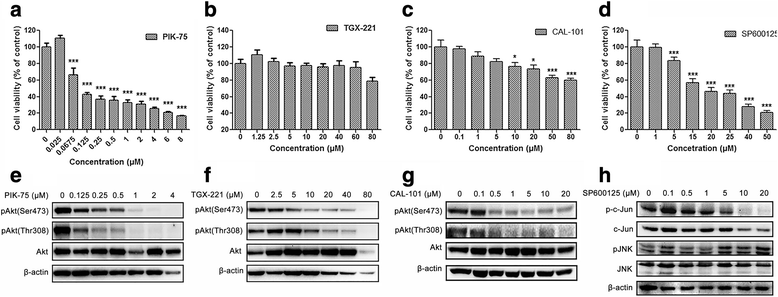
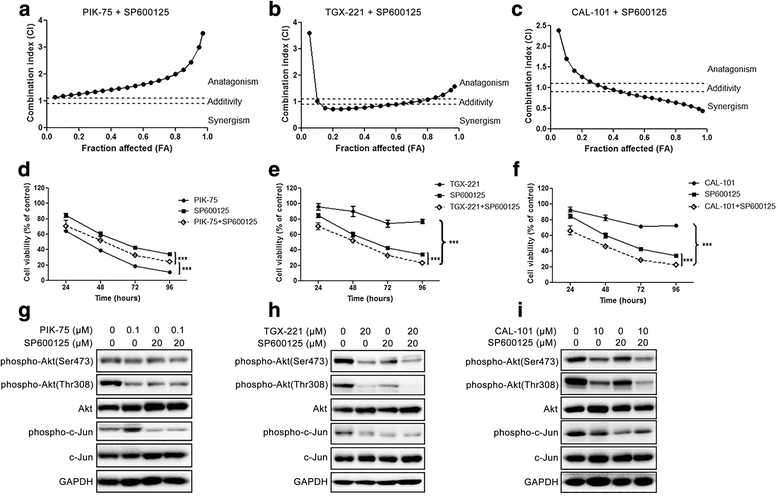
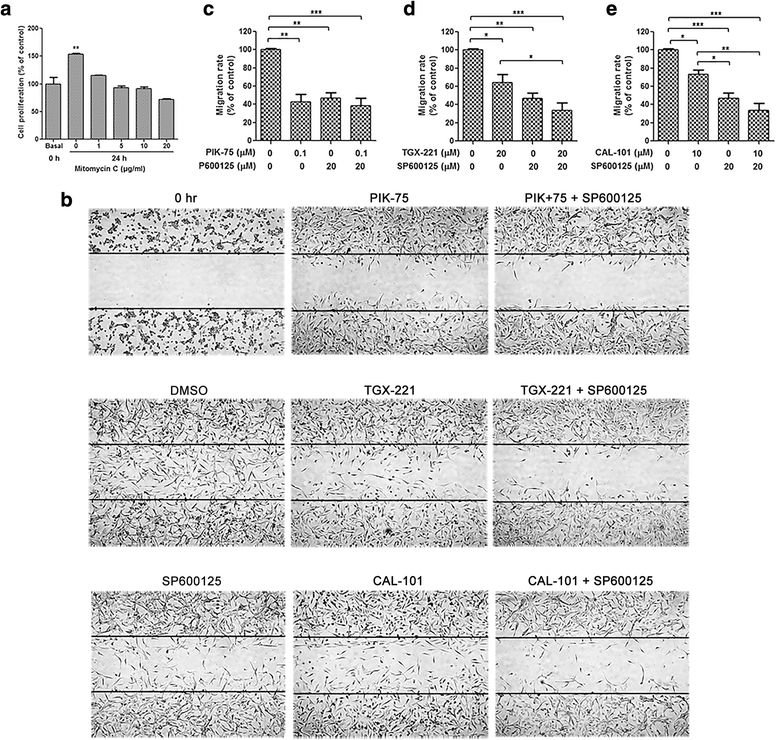
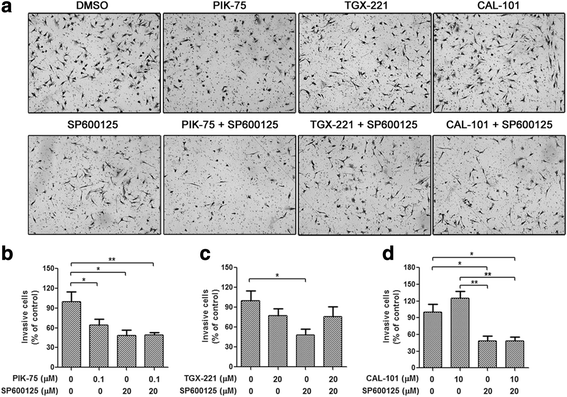
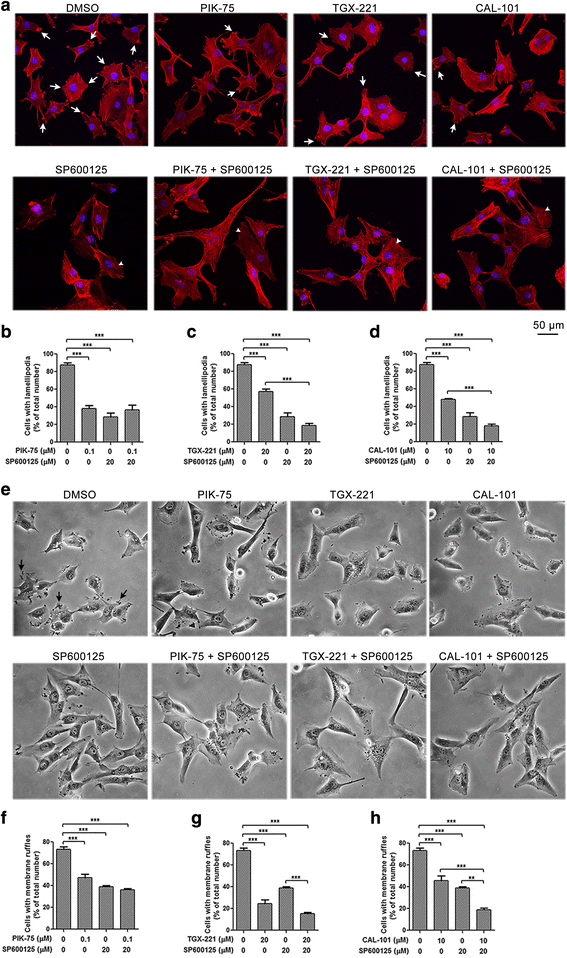
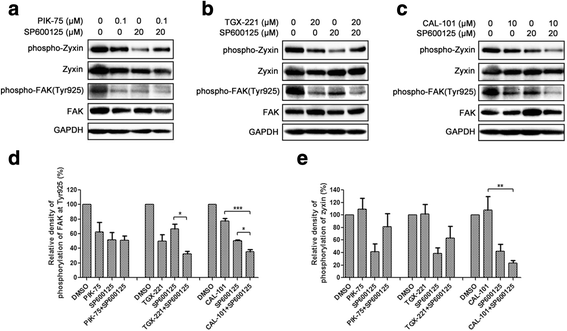
Results: We showed that combined inhibition of these PI3K isoforms and JNK exerted divergent effects on the proliferation, migration and invasion of glioblastoma cells in vitro. Pharmacological inhibition of p110β or p110δ, but not p110α, displayed synergistic inhibitory effect with JNK inhibition on glioblastoma cell proliferation and migration through decreasing phosphorylation of Akt, FAK and zyxin, leading to blockade of lamellipodia and membrane ruffles formation. No synergistic effect on invasion was observed in all the combination treatment. In vivo, combination of p110β and JNK inhibitors significantly reduced xenograft tumor growth compared with single inhibitor alone.
Conclusion: Concurrent inhibition of p110β and JNK exhibited synergistic effects on suppressing glioblastoma cell proliferation and migration in vitro and xenograft tumor growth in vivo. Our data suggest that combined inhibition of PI3K p110β isoform and JNK may serve as a potent and promising therapeutic approach for glioblastoma multiforme.
Keywords: Glioblastoma; JNK; Migration; PI3K; Proliferation; Synergism; p110β.
Figures

References
-
- Usatyuk PV, Fu P, Mohan V, Epshtein Y, Jacobson JR, Gomez-Cambronero J, Wary KK, Bindokas V, Dudek SM, Salgia R, et al. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J Biol Chem. 2014;289:13476–91. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

