Botulinum toxin type B for cervical dystonia
- PMID: 27176573
- PMCID: PMC8552447
- DOI: 10.1002/14651858.CD004315.pub3
Botulinum toxin type B for cervical dystonia
Abstract
Background: This is an update of a Cochrane review first published in 2004, and previously updated in 2009 (no change in conclusions). Cervical dystonia is a frequent and disabling disorder characterised by painful involuntary head posturing. Botulinum toxin type A (BtA) is usually considered the first line therapy for this condition, although botulinum toxin type B (BtB) is an alternative option.
Objectives: To compare the efficacy, safety and tolerability of botulinum toxin type B (BtB) versus placebo in people with cervical dystonia.
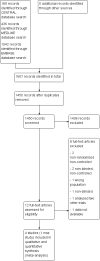
Search methods: We identified studies for inclusion in the review using the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, reference lists of articles and conference proceedings, last run in October 2015. We ran the search from 1977 to 2015. The search was unrestricted by language.
Selection criteria: Double-blind, parallel, randomised, placebo-controlled trials (RCTs) of BtB versus placebo in adults with cervical dystonia.
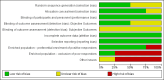
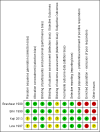
Data collection and analysis: Two independent authors assessed records, selected included studies, extracted data using a paper pro forma and evaluated the risk of bias. We resolved disagreements by consensus or by consulting a third author. We performed one meta-analysis for the comparison BtB versus placebo. We used random-effects models when there was heterogeneity and fixed-effect models when there was no heterogeneity. In addition, we performed pre-specified subgroup analyses according to BtB doses and BtA previous clinical responsiveness. The primary efficacy outcome was overall improvement on any validated symptomatic rating scale. The primary safety outcome was the number of participants with any adverse event.
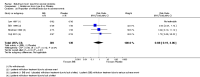
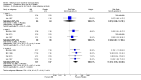
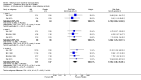
Main results: We included four RCTs of moderate overall methodological quality, including 441 participants with cervical dystonia. Three studies excluded participants known to have poorer response to Bt treatment, therefore including an enriched population with a higher probability of benefiting from Bt treatment. None of the trials were independently funded. All RCTs evaluated the effect of a single Bt treatment session using doses between 2500 U and 10,000 U. BtB was associated with an improvement of 14.7% (95% CI 9.8% to 19.5) in the patients' baseline clinical status as assessed by investigators, with reduction of 6.8 points in the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-total score) at week 4 after injection (95% CI 4.54 to 9.01). Mean difference (MD) in TWSTRS-pain score at week 4 was 2.20 (95% CI 1.25 to 3.15). Overall, both participants and clinicians reported an improvement of subjective clinical status. There were no differences between groups in the withdrawals rate due to adverse events or in the proportion of participants with adverse events. However, BtB-treated patients had a 7.65 (95% CI 2.75 to 21.32) and a 6.78 (95% CI 2.42 to 19.05) increased risk of treatment-related dry mouth and dysphagia, respectively. Statistical heterogeneity between studies was low to moderate for most outcomes. All tested dosages were efficacious against placebo without clear-cut evidence of a dose-response gradient. However, duration of effect (time until return to baseline TWSTRS-total score) and risk of dry mouth and dysphagia were greater in the subgroup of participants treated with higher BtB doses. Subgroup analysis showed a higher improvement with BtB among BtA-non-responsive participants, although there were no differences in the effect size between the BtA-responsive and non-responsive subgroups.
Authors' conclusions: A single BtB-treatment session is associated with a significant and clinically relevant reduction of cervical dystonia impairment including severity, disability and pain, and is well tolerated, when compared with placebo. However, BtB-treated patients are at an increased risk of dry mouth and dysphagia. There are no data from RCTs evaluating the effectiveness and safety of repeated BtB injection cycles. There are no RCT data to allow us to draw definitive conclusions on the optimal treatment intervals and doses, usefulness of guidance techniques for injection, and impact on quality of life.
Conflict of interest statement
Costa J, Ferreira JJ, Sampaio C have been investigators in clinical trials sponsored by Elan, Allergan, and Ipsen. Ferreira JJ and Sampaio C were speakers in symposia promoted by Elan, Allergan, and Ipsen. Moore AP has received fees from various companies marketing botulinum toxin for speaking at meetings and for advice. His unit has received funds for research.
Figures




























Update of
-
Botulinum toxin type B for cervical dystonia.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004315. doi: 10.1002/14651858.CD004315.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2016 May 13;(5):CD004315. doi: 10.1002/14651858.CD004315.pub3. PMID: 15674941 Updated.
References
References to studies included in this review
Brashear 1999 {published data only}
-
- Brashear A, Lew MF, Dykstra DD, Comella CL, Factor SA, Rodnitzky RL, et al. Safety and efficacy of NeuroBloc (Botulinum toxin type B) in type A‐responsive cervical dystonia. Neurology 1999;52 (Suppl 2):A292 (S46.003). - PubMed
-
- Brashear A, Lew MF, Dykstra DD, Comella CL, Factor SA, Rodnitzky RL, et al. Safety and efficacy of NeuroBloc (Botulinum toxin type B) in type A‐responsive cervical dystonia. Neurology 1999;53(7):1439‐46. - PubMed
-
- Factor S. Safety and efficacy of Neurobloc (Botulinum toxin type‐B) in type‐A responsive and type‐A resistant patients with cervical dystonia. Toxins'99 (www.wemove.org). 1999:9.
-
- Factor S, Adler CH, Brashear A, Brin MF, Comella CL, Dykstra DD, et al. Safety and efficacy of Neurobloc (Botulinum toxin type‐B) in type‐A responsive and type‐A resistant patients with cervical dystonia. Movement Disorders 2000;15 (Suppl 2):7.
-
- Koller M, Wallace JD, Willmer‐Hulme A, Chiang P, Murray JJ. Evaluation of Neurobloc (Botulinum toxin type B) efficacy in patients with cervical dystonia. Movement Disorders 2000;15 (Suppl 2):31.
Brin 1999 {published data only}
-
- Brin MF, Lew MF, Adler CH, Comella CL, Factor SA, Jankovic J, et al. Safety and efficacy of NeuroBloc (Botulinum toxin type B) in type A‐resistant cervical dystonia. Neurology 1999;22;53(7):1431‐8. - PubMed
-
- Brin MF, Lew MF, Adler CH, Comella CL, Factor SA, Jankovic J, et al. Safety and efficacy of NeuroBloc (Botulinum toxin type B) in type A‐resistant cervical dystonia. Neurology 1999;52 (Suppl 2):A293 (S46.004). - PubMed
-
- Factor S. Safety and efficacy of Neurobloc (Botulinum toxin type‐B) in type‐A responsive and type‐A resistant patients with cervical dystonia. Neurology 1999;7:1431‐8. - PubMed
-
- Factor S, Adler CH, Brashear A, Brin MF, Comella CL, Dykstra DD, et al. Safety and efficacy of Neurobloc (Botulinum toxin type‐B) in type‐A responsive and type‐A resistant patients with cervical dystonia. Movement Disorders 2000;15 (Suppl 2):7.
-
- Koller M, Wallace JD, Willmer‐Hulme A, Chiang P, Murray JJ. Evaluation of Neurobloc (Botulinum toxin type B) efficacy in patients With cervical dystonia. Movement Disorders. 2000; Vol. 15 (Suppl 2):31.
Kaji 2013 {published data only}
-
- Kaji R, Shimizu H, Takase T, Osawa M, Yanagisawa N. A double‐blind comparative study to evaluate the efficacy and safety of NerBloc (rimabotulinumtoxinB) administered in a single dose to patients with cervical dystonia. Brain and Nerve 2013;65(2):203‐11. - PubMed
Lew 1997 {published data only}
-
- Koller M, Wallace JD, Willmer‐Hulme A, Chiang P, Murray JJ. Evaluation of Neurobloc (Botulinum toxin type B) efficacy in patients with cervical dystonia. Movement Disorders 2000;15 (Suppl 2):31.
-
- Lew MF, Adornato BT, Duane DD, Dykstra DD, Factor SA, Massey JM, et al. Botulinum toxin type B: a double‐blind, placebo controlled, safety and efficacy study in cervical dystonia. Neurology 1997;49:701‐7. - PubMed
-
- Lew MF, Brashear A, Factor S. The safety and efficacy of Botulinum toxin type B in the treatment of patients with cervical dystonia: summary of three controlled clinical trials. Neurology 2000;55 (Suppl 5):S29‐S35. - PubMed
References to studies excluded from this review
AN072‐008 1995 {published data only}
-
- American BotB Cervical Dystonia Study Group. BotB (Botulinum toxin type B) in the treatment of cervical dystonia (CD) ‐ protocol AN072‐008: an interim analysis. Movement Disorders 1995;10:2874 (abstract).
-
- Koller M, Wallace JD, Willmer‐Hulme A, Chiang P, Murray JJ. Evaluation of Neurobloc (Botulinum toxin type B) efficacy in patients with cervical dystonia. Movement Disorders 2000;15 (Suppl 2):31.
Chinnapongse 2010 {published data only}
-
- Chinnapongse R, Pappert EJ, Evatt M, Freeman A, Birmingham W. An open‐label, sequential dose‐escalation, safety, and tolerability study of rimabotulinumtoxinB in subjects with cervical dystonia. International Journal of Neuroscience 2010;120(11):703‐10. - PubMed
Cullis 2000 {published data only}
-
- Cullis PA, Barnes M, Duane D, Chen RE, Freeman A, Fross R, et al. Safety and tolerability of repeated doses of Neurobloc (Botulinum toxin type B) in patients with cervical dystonia: an open‐label, dose‐escalation study. Movement Disorders 2000;15 (Suppl 2):29.
Dressler 2005 {published data only}
-
- Dressler D, Bigalke H. Botulinum toxin type B de novo therapy of cervical dystonia: frequency of antibody induced therapy failure . Journal of neurology 2005;252(8):904‐7. - PubMed
Jacob 2003 {published data only}
-
- Jacob CI. Botulinum toxin type B ‐ onset, duration, and efficacy: comparing dilution with preserved versus nonpreserved saline. Cosmetic Dermatology 2003;16(7):25.
Jankovic 2006 {published data only}
-
- Jankovic J, Hunter C, Dolimbek DZ, Dolimbek GS, Adler CH, Brashear A, et al. Clinico‐immunologic aspects of botulinum toxin type B treatment of cervical dystonia. Neurology 2006;67(12):2233‐5. - PubMed
Lew 2002 {published data only}
-
- Lew MF. Botulinum toxin type B: An effective treatment for alleviating pain associated with cervical dystonia. Journal of Back and Musculoskeletal Rehabilitation 2002;16(1):3‐9. - PubMed
Truong 1997 {published data only}
-
- Truong DD, Cullis PA, O'Brien CF, Koller M, Villegas TP, Wallace JD. BotB (Botulinum toxin type B): Evaluation of safety and tolerability in Botulinum type A‐resistant cervical dystonia patients (preliminary study). Movement Disorders 1997;12(5):772‐5. - PubMed
Additional references
Abrams 2005
-
- Abrams KR, Gillies CL, Lambert PC. Meta‐analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823‐44. - PubMed
Albanese 2013
Altman 2002
Antonucci 2008
Balint 2015
-
- Balint B, Bhatia KP. Isolated and combined dystonia syndromes ‐ an update on new genes and their phenotypes. European Journal of Neurology 2015;22(4):610‐7. - PubMed
Benecke 2012
Boroff 1975
-
- Boroff DA, Chen GS. On the question of permeability of the blood–brain barrier to botulinum toxin. Int Arch Allergy ApplImmunol. 1975;48:495–504. - PubMed
Brashear 2008
-
- Brashear A. Botulinum toxin serotype A for cervical dystonia — an assessment. US Neurol 2008;4(2):58‐61.
Brin 2008
-
- Brin MF, Comella CL, Jankovic J, Lai F, Naumann M, CD‐017 BoNTA Study Group. Long‐term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Movement Disorders 2008 Jul;23(10):1353‐60. - PubMed
Brozek 2006
Chan 1991
-
- Chan J, Brin MF, Fanh S. Idiopathic cervical dystonia: clinical characteristics. Movement Disorders 1991;6:119‐26. - PubMed
Cohen 1988
-
- Cohen J. Statistical power analysis in the behavioral sciences. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
Comella 2015
Consky 1994
-
- Consky ES, Lang AE. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M editor(s). Therapy with botulinum toxin. New York, NY: Marcel Dekker, Inc, 1994:211‐37.
de Paiva 1999
Defazio 2013
Duchen 1971
-
- Duchen LW. An electron microscopic study of the changes induced by botulinum toxin in the motor end‐plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971;14:47‐60. - PubMed
Edwards 2000
-
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000 Oct 7;356(9237):1255‐1259. - PubMed
Eleopra 1997
-
- Eleopra R, Tugnoli V, Rossetto O, Montecucco C, Grandis D. Botulinum neurotoxin serotype C: a novel effective botulinum toxin therapy in human. Neuroscience Letters 1997;224(2):91‐4. - PubMed
ESDE 2000
-
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries . Journal of Neurology 2000;247:787‐92. - PubMed
Fabbri 2015
-
- Fabbri M, Leodori G, Fernandes RM, Bhidayasiri R, Marti MJ, Colosimo C, et al. Neutralizing antibody and Botulinum toxin therapy: a sSystematic review and meta‐analysis. Neurotox Res 2015;29:105‐117. [PUBMED: 26467676] - PubMed
Filippi 1993
-
- Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113:400–4. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐773. - PubMed
Foltz 1959
-
- Foltz EL, Knopp LM, Ward AA. Experimental spasmodic torticollis. Journal of Neurosurgery 1959;16:55‐72. - PubMed
Frevert 2010
Greene 1993
-
- Greene PE, Fahn S. Use of botulinum toxin type F injections to treat torticollis in patients with immunity to botulinum toxin type A. Movement Disorders 1993;8(4):479‐83. - PubMed
Hallett 1998
-
- Hallett M. The neurophysiology of dystonia. Arch Neurol 1998 May;55(5):601‐603. - PubMed
Hanna 1998
-
- Hanna PA, Jankovic J. Mouse bioassay versus Western blot assay for botulinum toxin antibodies: correlation with clinical response. Neurology 1998 Jun;50(6):1624‐9. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holland 1981
-
- Holland RL, Brown MC. Nerve growth in botulinum toxin poisoned muscles. Neuroscience. 1981;6:1167–79. - PubMed
Jahnanshani 1990
-
- Jahnanshani M, Marion M‐H, Marsden CD. Natural history of adult‐onset idiopathic torticollis. Archives of Neurology 1990;47:548‐52. - PubMed
Jankovic 2004
Juzans 1996
-
- Juzans P, Comella J, Molgo J, Faille L, Angaut‐Petit D. Nerve terminal sprouting inbotulinum type‐A treated mouse levator auris longus muscle. Neuromuscul Disord. 1996;6(3):177‐85. - PubMed
Lange 2009
-
- Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing?. Clin Neuropharmacol 2009 Jul‐Aug;32(4):213‐8. - PubMed
Macefield 2014
Matak 2014
-
- Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014 Aug‐Sep;119‐120:39–59. - PubMed
Matak 2015
-
- Matak I, Lacković Z. Botulinum neurotoxin type A: Actions beyond SNAP‐25?. Toxicology. 2015;335:79‐84. - PubMed
Michiels 2005
-
- Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta‐analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care 2005;21(1):119‐25. - PubMed
Muller 2009
-
- Müller K, Mix E, Adib Saberi F, Dressler D, Benecke R. Prevalence of neutralising antibodies in patients treated with botulinum toxin type A for spasticity. J Neural Transm 2009 May;116(5):579‐85. - PubMed
Palomar 2012
-
- Palomar FJ, Mir P. Neurophysiological changes after intramuscular injection of botulinum toxin. Clin Neurophysiol. 2012;123:23:54–60. - PubMed
Pellizzari 1999
Peters 2006
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA. 2006;295(6):676‐80. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Centre, The Cochrane Collaboration, 2014.
Rosales 1996
-
- Rosales RL, Arimura K, Takenaka S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin‐A injection. Muscle Nerve. 1996;19:488–96. - PubMed
Rosales 2010
-
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. Eur J Neurol. 2010;17:71–80. - PubMed
Simpson 2004
-
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167‐93. - PubMed
Smeeth 1999
Steeves 2012
-
- Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: A systematic review and meta‐analysis. Movement Disorders. 2012 Dec;27(14):1789‐96. - PubMed
Sterne 2001
-
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046‐1055. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tugwell 2007
Walker 2014
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

