The catalytic power of magnesium chelatase: a benchmark for the AAA(+) ATPases
- PMID: 27176620
- PMCID: PMC4982103
- DOI: 10.1002/1873-3468.12214
The catalytic power of magnesium chelatase: a benchmark for the AAA(+) ATPases
Abstract
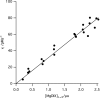
In the first committed reaction of chlorophyll biosynthesis, magnesium chelatase couples ATP hydrolysis to the thermodynamically unfavorable Mg(2+) insertion into protoporphyrin IX (ΔG°' of circa 25-33 kJ·mol(-1) ). We explored the thermodynamic constraints on magnesium chelatase and demonstrate the effect of nucleotide hydrolysis on both the reaction kinetics and thermodynamics. The enzyme produces a significant rate enhancement (kcat /kuncat of 400 × 10(6) m) and a catalytic rate enhancement, kcat/KmDIXK0.5Mgkuncat, of 30 × 10(15) m(-1) , increasing to 300 × 10(15) m(-1) with the activator protein Gun4. This is the first demonstration of the thermodynamic benefit of ATP hydrolysis in the AAA(+) family.
Keywords: ATP hydrolysis; ATPases associated with various cellular activities (AAA) magnesium protoporphyrin IX; Gun4; chelatase; chlorophyll biosynthesis.
© 2016 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures


References
-
- Reid JD and Hunter CN (2004) Magnesium‐dependent ATPase activity and cooperativity of magnesium chelatase from Synechocystis sp. PCC6803. J Biol Chem 279, 26893–26899. - PubMed
-
- Sawicki A and Willows RD (2008) Kinetic analyses of the magnesium chelatase provide insights into the mechanism, structure and formation of the complex. J Biol Chem 283, 31294–31302. - PubMed
-
- Jensen PE, Gibson LCD and Hunter CN (1999) ATPase activity associated with the magnesium‐protoporphyrin IX chelatase enzyme of Synechocystis sp. PCC6803: evidence for ATP hydrolysis during Mg2+ insertion, and the MgATP‐dependent interaction of the ChlI and ChlD subunits. Biochem J 339, 127–134. - PMC - PubMed
-
- Reid JD, Siebert CA, Bullough PA and Hunter CN (2003) The ATPase activity of the ChlI subunit of magnesium chelatase and formation of a heptameric AAA+ ring. Biochemistry 42, 6912–6920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

