The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma
- PMID: 27177573
- PMCID: PMC4933490
- DOI: 10.1093/neuonc/now102
The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma
Abstract
Background: There is strong concern about the costs associated with adding tumor-treating fields (TTF) therapy to standard first-line treatment for glioblastoma (GBM). Hence, we aimed to determine the cost-effectiveness of TTF therapy for the treatment of newly diagnosed patients with GBM.
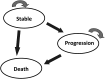
Methods: We developed a 3-health-state Markov model. The perspective was that of the French Health Insurance, and the horizon was lifetime. We calculated the transition probabilities from the survival parameters reported in the EF-14 trial. The main outcome measure was incremental effectiveness expressed as life-years gained (LYG). Input costs were derived from the literature. We calculated the incremental cost-effectiveness ratio (ICER) expressed as cost/LYG. We used 1-way deterministic and probabilistic sensitivity analysis to evaluate the model uncertainty.
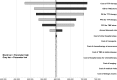
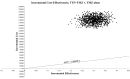
Results: In the base-case analysis, adding TTF therapy to standard of care resulted in increases of life expectancy of 4.08 months (0.34 LYG) and €185 476 per patient. The ICER was €549 909/LYG. The discounted ICER was €596 411/LYG. Parameters with the most influence on ICER were the cost of TTF therapy, followed equally by overall survival and progression-free survival in both arms. The probabilistic sensitivity analysis showed a 95% confidence interval of the ICER of €447 017/LYG to €745 805/LYG with 0% chance to be cost-effective at a threshold of €100 000/LYG.
Conclusion: The ICER of TTF therapy at first-line treatment is far beyond conventional thresholds due to the prohibitive announced cost of the device. Strong price regulation by health authorities could make this technology more affordable and consequently accessible to patients.
Keywords: brain tumor; cost-effectiveness analysis; glioblastoma; temozolomide; tumor-treating fields.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Living in a material world: tumor-treating fields at the top of the charts.Neuro Oncol. 2016 Aug;18(8):1033-4. doi: 10.1093/neuonc/now138. Neuro Oncol. 2016. PMID: 27382117 Free PMC article. No abstract available.
Similar articles
-
Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model.J Neurooncol. 2019 Jul;143(3):605-611. doi: 10.1007/s11060-019-03197-w. Epub 2019 May 24. J Neurooncol. 2019. PMID: 31127507
-
Cost-effectiveness of the long-term use of temozolomide for treating newly diagnosed glioblastoma in Germany.J Neurooncol. 2018 Jun;138(2):359-367. doi: 10.1007/s11060-018-2804-x. Epub 2018 Feb 21. J Neurooncol. 2018. PMID: 29468446 Clinical Trial.
-
Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: a cost-effectiveness study.J Med Econ. 2019 Oct;22(10):1006-1013. doi: 10.1080/13696998.2019.1614933. Epub 2019 May 20. J Med Econ. 2019. PMID: 31050315
-
Economic Evaluation of Bevacizumab for the First-Line Treatment of Newly Diagnosed Glioblastoma Multiforme.J Clin Oncol. 2015 Jul 10;33(20):2296-302. doi: 10.1200/JCO.2014.59.7245. Epub 2015 May 26. J Clin Oncol. 2015. PMID: 26014296 Review.
-
Cost effectiveness of prostacyclins in pulmonary arterial hypertension.Appl Health Econ Health Policy. 2012 May 1;10(3):175-88. doi: 10.2165/11630780-000000000-00000. Appl Health Econ Health Policy. 2012. PMID: 22452448 Review.
Cited by
-
Therapeutic cell-based vaccines for glioblastoma multiforme.Med Oncol. 2023 Nov 12;40(12):354. doi: 10.1007/s12032-023-02220-5. Med Oncol. 2023. PMID: 37952224 Review.
-
Cost-effectiveness analysis of 11 pharmacotherapies for recurrent glioblastoma in the USA and China.Ther Adv Med Oncol. 2024 Jul 31;16:17588359241264727. doi: 10.1177/17588359241264727. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39091601 Free PMC article.
-
Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model.J Neurooncol. 2019 Jul;143(3):605-611. doi: 10.1007/s11060-019-03197-w. Epub 2019 May 24. J Neurooncol. 2019. PMID: 31127507
-
Tumour treating fields therapy for glioblastoma: current advances and future directions.Br J Cancer. 2021 Feb;124(4):697-709. doi: 10.1038/s41416-020-01136-5. Epub 2020 Nov 4. Br J Cancer. 2021. PMID: 33144698 Free PMC article. Review.
-
Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma.Neurol Med Chir (Tokyo). 2018 Oct 15;58(10):405-421. doi: 10.2176/nmc.ra.2018-0141. Epub 2018 Sep 21. Neurol Med Chir (Tokyo). 2018. PMID: 30249919 Free PMC article. Review.
References
-
- Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis ESMO Guidelines Working Group. . High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii93–ii101. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Chinot OL, Wick W, Mason W et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

