Functional Plasticity of Adipose-Derived Stromal Cells During Development of Obesity
- PMID: 27177576
- PMCID: PMC4922846
- DOI: 10.5966/sctm.2015-0240
Functional Plasticity of Adipose-Derived Stromal Cells During Development of Obesity
Abstract
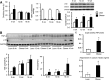
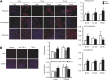
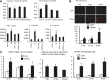
Obesity is a major risk factor for a number of chronic diseases, including diabetes, cardiovascular diseases, and cancer. Expansion of the adipose mass requires adipocyte precursor cells that originate from multipotent adipose-derived stromal cells (ASCs), which in turn also participate in repair activities. ASC function might decline in a disease milieu, but it remains unclear whether ASC function varies during the development of obesity. We tested the hypothesis that microenvironmental inflammatory changes during development of metabolic disorders in obesity affect ASC function. Domestic pigs were fed with an atherogenic (n = 7) or normal (n = 7) diet for 16 weeks. Abdominal adipose tissue biopsies were collected after 8, 12, and 16 weeks of diet for ASC isolation and immunohistochemistry of in situ ASCs and tumor necrosis factor-α (TNF-α). Longitudinal changes in proliferation, differentiation, and anti-inflammatory functions of ASCs were assessed. At 16 weeks, upregulated TNF-α expression in adipose tissue from obese pigs was accompanied by increased numbers of adipocyte progenitors (CD24(+)/CD34(+)) in adipose tissue and enlarged adipocyte size. In vitro, ASCs from obese pigs showed enhanced adipogenic and osteogenic propensity, which was abolished by anti-TNF-α treatment, whereas lean ASCs treated with TNF-α showed enhanced adipogenesis. Furthermore, obese ASCs showed increased senescence compared with lean ASCs, whereas their immunomodulatory capacity was preserved. Adipose tissue inflammation promotes an increase in resident adipocyte progenitors and upregulated TNF-α enhances ASC adipogenesis. Thus, adipose tissue anti-inflammatory strategies might be a novel target to attenuate obesity and its complications.
Significance: Adipose-derived stromal cell (ASC) function might decline in a disease milieu, but it remains unclear whether ASC function varies during the development of obesity. This study tested the hypothesis that microenvironmental inflammatory changes during development of metabolic disorders in obesity affect ASC function. It was found that ASCs show increased propensity for differentiation into adipocytes, which is partly mediated by upregulated tumor necrosis factor-α (TNF-α), likely in their adipose tissue microenvironment. Furthermore, TNF-α magnified obese ASC senescence, although it did not regulate their anti-inflammatory properties. Thus, adipose tissue inflammation might be a novel therapeutic target to avert ASC maldifferentiation and senescence.
Keywords: Adipose tissue; Inflammation; Mesenchymal stem cells; Obesity.
©AlphaMed Press.
Figures
Similar articles
-
Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue.Stem Cells Transl Med. 2016 Apr;5(4):464-75. doi: 10.5966/sctm.2015-0161. Epub 2016 Mar 8. Stem Cells Transl Med. 2016. PMID: 26956208 Free PMC article.
-
Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function.Cardiovasc Res. 2017 Jul 1;113(9):1064-1073. doi: 10.1093/cvr/cvx096. Cardiovasc Res. 2017. PMID: 28498891 Review.
-
Adipose tissue mitochondrial dysfunction in human obesity is linked to a specific DNA methylation signature in adipose-derived stem cells.Int J Obes (Lond). 2019 Jun;43(6):1256-1268. doi: 10.1038/s41366-018-0219-6. Epub 2018 Sep 27. Int J Obes (Lond). 2019. PMID: 30262812 Free PMC article.
-
Serial changes in the proliferation and differentiation of adipose-derived stem cells after ionizing radiation.Stem Cell Res Ther. 2016 Aug 17;7(1):117. doi: 10.1186/s13287-016-0378-0. Stem Cell Res Ther. 2016. PMID: 27530249 Free PMC article.
-
Adipose Stromal Cell Expansion and Exhaustion: Mechanisms and Consequences.Cells. 2020 Apr 2;9(4):863. doi: 10.3390/cells9040863. Cells. 2020. PMID: 32252348 Free PMC article. Review.
Cited by
-
Effects of obesity on reparative function of human adipose tissue-derived mesenchymal stem cells on ischemic murine kidneys.Int J Obes (Lond). 2022 Jun;46(6):1222-1233. doi: 10.1038/s41366-022-01103-5. Epub 2022 Mar 7. Int J Obes (Lond). 2022. PMID: 35256761 Free PMC article.
-
Metabolic Syndrome Induces Epigenetic Alterations in Mitochondria-Related Genes in Swine Mesenchymal Stem Cells.Cells. 2023 Apr 27;12(9):1274. doi: 10.3390/cells12091274. Cells. 2023. PMID: 37174674 Free PMC article.
-
Metabolic Syndrome Modulates Protein Import into the Mitochondria of Porcine Mesenchymal Stem Cells.Stem Cell Rev Rep. 2019 Jun;15(3):427-438. doi: 10.1007/s12015-018-9855-4. Stem Cell Rev Rep. 2019. PMID: 30338499 Free PMC article.
-
GPX7 Facilitates BMSCs Osteoblastogenesis via ER Stress and mTOR Pathway.J Cell Mol Med. 2021 Nov;25(22):10454-10465. doi: 10.1111/jcmm.16974. Epub 2021 Oct 9. J Cell Mol Med. 2021. PMID: 34626080 Free PMC article.
-
Human Obesity Induces Dysfunction and Early Senescence in Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells.Front Cell Dev Biol. 2020 Mar 26;8:197. doi: 10.3389/fcell.2020.00197. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32274385 Free PMC article.
References
-
- Eckel RH. Obesity and heart disease: A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96:3248–3250. - PubMed
-
- Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–1437. - PubMed
-
- Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. - PubMed
Publication types
MeSH terms
Grants and funding
- DK106427/DK/NIDDK NIH HHS/United States
- K23 DK109134/DK/NIDDK NIH HHS/United States
- R01 HL123160/HL/NHLBI NIH HHS/United States
- K08 DK106427/DK/NIDDK NIH HHS/United States
- DK73608/DK/NIDDK NIH HHS/United States
- HL123160/HL/NHLBI NIH HHS/United States
- DK102325/DK/NIDDK NIH HHS/United States
- DK104273/DK/NIDDK NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- R01 DK073608/DK/NIDDK NIH HHS/United States
- U01 DK104273/DK/NIDDK NIH HHS/United States
- R01 DK102325/DK/NIDDK NIH HHS/United States
- UL1-TR000135/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

