Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium
- PMID: 27177577
- PMCID: PMC4922855
- DOI: 10.5966/sctm.2015-0305
Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium
Abstract
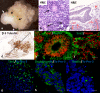
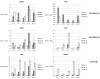
Tissue organoids are a promising technology that may accelerate development of the societal and NIH mandate for precision medicine. Here we describe a robust and simple method for generating cerebral organoids (cOrgs) from human pluripotent stem cells by using a chemically defined hydrogel material and chemically defined culture medium. By using no additional neural induction components, cOrgs appeared on the hydrogel surface within 10-14 days, and under static culture conditions, they attained sizes up to 3 mm in greatest dimension by day 28. Histologically, the organoids showed neural rosette and neural tube-like structures and evidence of early corticogenesis. Immunostaining and quantitative reverse-transcription polymerase chain reaction demonstrated protein and gene expression representative of forebrain, midbrain, and hindbrain development. Physiologic studies showed responses to glutamate and depolarization in many cells, consistent with neural behavior. The method of cerebral organoid generation described here facilitates access to this technology, enables scalable applications, and provides a potential pathway to translational applications where defined components are desirable.
Significance: Tissue organoids are a promising technology with many potential applications, such as pharmaceutical screens and development of in vitro disease models, particularly for human polygenic conditions where animal models are insufficient. This work describes a robust and simple method for generating cerebral organoids from human induced pluripotent stem cells by using a chemically defined hydrogel material and chemically defined culture medium. This method, by virtue of its simplicity and use of defined materials, greatly facilitates access to cerebral organoid technology, enables scalable applications, and provides a potential pathway to translational applications where defined components are desirable.
Keywords: Adrenoleukodystrophy; Brain; In vitro techniques; Organoids; Stem cells.
©AlphaMed Press.
Figures


Similar articles
-
Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles.Cells. 2020 May 23;9(5):1301. doi: 10.3390/cells9051301. Cells. 2020. PMID: 32456176 Free PMC article.
-
Clinically Amendable, Defined, and Rapid Induction of Human Brain Organoids from Induced Pluripotent Stem Cells.Methods Mol Biol. 2019;1576:13-22. doi: 10.1007/7651_2017_95. Methods Mol Biol. 2019. PMID: 29119484
-
Three-dimensional in vitro tissue culture models of brain organoids.Exp Neurol. 2021 May;339:113619. doi: 10.1016/j.expneurol.2021.113619. Epub 2021 Jan 23. Exp Neurol. 2021. PMID: 33497645 Review.
-
3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders.J Biomed Sci. 2017 Aug 20;24(1):59. doi: 10.1186/s12929-017-0362-8. J Biomed Sci. 2017. PMID: 28822354 Free PMC article. Review.
-
Scalable Generation of Mature Cerebellar Organoids from Human Pluripotent Stem Cells and Characterization by Immunostaining.J Vis Exp. 2020 Jun 13;(160). doi: 10.3791/61143. J Vis Exp. 2020. PMID: 32597849
Cited by
-
From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish.Int J Mol Sci. 2021 Dec 7;22(24):13180. doi: 10.3390/ijms222413180. Int J Mol Sci. 2021. PMID: 34947977 Free PMC article. Review.
-
Polymer design of microwell hydrogels influences epithelial-mesenchymal interactions during human bronchosphere formation.Adv Nanobiomed Res. 2025 Jan;5(1):2300110. doi: 10.1002/anbr.202300110. Epub 2024 Nov 21. Adv Nanobiomed Res. 2025. PMID: 40740547
-
Cancer patient-derived organoids: Novel models for the study of natural products.Int J Biol Sci. 2025 Jul 11;21(10):4485-4503. doi: 10.7150/ijbs.114373. eCollection 2025. Int J Biol Sci. 2025. PMID: 40765827 Free PMC article. Review.
-
Building the brain from scratch: Engineering region-specific brain organoids from human stem cells to study neural development and disease.Curr Top Dev Biol. 2021;142:477-530. doi: 10.1016/bs.ctdb.2020.12.011. Epub 2021 Feb 18. Curr Top Dev Biol. 2021. PMID: 33706925 Free PMC article.
-
Human pluripotent stem cell-derived models and drug screening in CNS precision medicine.Ann N Y Acad Sci. 2020 Jul;1471(1):18-56. doi: 10.1111/nyas.14012. Epub 2019 Mar 15. Ann N Y Acad Sci. 2020. PMID: 30875083 Free PMC article. Review.
References
-
- Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: Self-organising stem cells. Development. 2012;139:4111–4121. - PubMed
-
- Karus M, Blaess S, Brüstle O. Self-organization of neural tissue architectures from pluripotent stem cells. J Comp Neurol. 2014;522:2831–2844. - PubMed
-
- Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014;345:1247125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

