The Planar Lipid Bilayer System Serves as a Reductionist Approach for Studying NK Cell Immunological Synapses and Their Functions
- PMID: 27177664
- PMCID: PMC5614496
- DOI: 10.1007/978-1-4939-3684-7_13
The Planar Lipid Bilayer System Serves as a Reductionist Approach for Studying NK Cell Immunological Synapses and Their Functions
Abstract
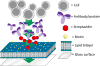
The immunological synapse (IS) is the junction between an immune cell (e.g., a T or NK cell) and another cell (e.g., an antigen-presenting cell (APC), or a tumor cell). The formation of the IS is crucial for cell-mediated immunity, and as such, an understanding of both the composition of the IS and the factors that drive its formation are essential for understanding how and when NK cells eliminate susceptible target cells. The supported lipid bilayer (SLB) system is a highly effective tool for directly studying the IS. SLBs confer three main advantages: (1) they allow for synapse formation on a level horizontal surface, allowing for direct visualization of the IS under high resolution imaging systems, (2) they mimic the surface of a target cell by providing a fluid mosaic into which surface proteins can be embedded while permitting free motion in two dimensions, which is important for studying the dynamics of synapse formation, and (3) they allow investigators to determine the exact composition of the bilayer, thus in turn allowing them to answer very specific questions about the IS. It is our hope that this chapter will furnish readers with an awareness of the applications of the SLB system for studying the IS in NK cells, and also of a basic knowledge of how to use this system for themselves.
Keywords: Confocal microscopy; IS; Immune synapse; Immunological synapse; Immunosynapse; NK; Natural killer; SLB; Supported lipid bilayer.
Figures





References
-
- Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–7. - PubMed
-
- McConnell HM, Watts TH, Weis RM, et al. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986;864(1):95–106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

