Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer
- PMID: 27177865
- PMCID: PMC6279075
- DOI: 10.1093/annonc/mdw204
Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer
Abstract
Background: We previously reported the safety of concurrent cetuximab, an antibody against epidermal growth factor receptor (EGFR), pemetrexed, and radiation therapy (RT) in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). In this non-comparative phase II randomized trial, we evaluated this non-platinum combination with or without bevacizumab, an inhibitor of vascular endothelial growth factor (VEGF).
Patients and methods: Patients with previously untreated stage III-IVB SCCHN were randomized to receive: conventionally fractionated radiation (70 Gy), concurrent cetuximab, and concurrent pemetrexed (arm A); or the identical regimen plus concurrent bevacizumab followed by bevacizumab maintenance for 24 weeks (arm B). The primary end point was 2-year progression-free survival (PFS), with each arm compared with historical control. Exploratory analyses included the relationship of established prognostic factors to PFS and quality of life (QoL).
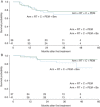
Results: Seventy-eight patients were randomized: 66 oropharynx (42 HPV-positive, 15 HPV-negative, 9 unknown) and 12 larynx; 38 (49%) had heavy tobacco exposure. Two-year PFS was 79% [90% confidence interval (CI) 0.69-0.92; P < 0.0001] for arm A and 75% (90% CI 0.64-0.88; P < 0.0001) for arm B, both higher than historical control. No differences in PFS were observed for stage, tobacco history, HPV status, or type of center (community versus academic). A significantly increased rate of hemorrhage occurred in arm B. SCCHN-specific QoL declined acutely, with marked improvement but residual symptom burden 1 year post-treatment.
Conclusions: RT with a concurrent non-platinum regimen of cetuximab and pemetrexed is feasible in academic and community settings, demonstrating expected toxicities and promising efficacy. Adding bevacizumab increased toxicity without apparent improvement in efficacy, countering the hypothesis that dual EGFR-VEGF targeting would overcome radiation resistance, and enhance clinical benefit. Further development of cetuximab, pemetrexed, and RT will require additional prospective study in defined, high-risk populations where treatment intensification is justified.
Keywords: bevacizumab; cetuximab; clinical trial; head and neck cancer; pemetrexed; radiation therapy.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Adelstein DJ, Li Y, Adams GL et al. . An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–98. - PubMed
-
- Bonner JA, Harari PM, Giralt J et al. . Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–578. - PubMed
-
- Rosenthal DI, Harari PM, Giralt J et al. . Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 Phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol 2016; 34: 1300–1308. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous