A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase
- PMID: 27178246
- PMCID: PMC4956176
- DOI: 10.1177/1744806916649192
A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase
Abstract
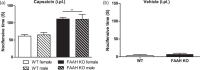
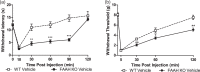
Fatty-acid amide hydrolase (FAAH) is the major enzyme responsible for degradation of anandamide, an endocannabinoid. Pharmacological inhibition or genetic deletion of FAAH (FAAH KO) produces antinociception in preclinical pain models that is largely attributed to anandamide-induced activation of cannabinoid receptors. However, FAAH metabolizes a wide range of structurally related, biologically active lipid signaling molecules whose functions remain largely unknown. Some of these endogenous lipids, including anandamide itself, may exert pro-nociceptive effects under certain conditions. In our study, FAAH KO mice exhibited a characteristic analgesic phenotype in the tail flick test and in both formalin and carrageenan models of inflammatory nociception. Nonetheless, intradermal injection of the transient receptor potential channel V1 (TRPV1) agonist capsaicin increased nocifensive behavior as well as mechanical and heat hypersensitivity in FAAH KO relative to wild-type mice. This pro-nociceptive phenotype was accompanied by increases in capsaicin-evoked Fos-like immunoreactive (FLI) cells in spinal dorsal horn regions implicated in nociceptive processing and was attenuated by CB1 (AM251) and TRPV1 (AMG9810) antagonists. When central sensitization was established, FAAH KO mice displayed elevated levels of anandamide, other fatty-acid amides, and endogenous TRPV1 agonists in both paw skin and lumbar spinal cord relative to wild-type mice. Capsaicin decreased spinal cord 2-AG levels and increased arachidonic acid and prostaglandin E2 levels in both spinal cord and paw skin irrespective of genotype. Our studies identify a previously unrecognized pro-nociceptive phenotype in FAAH KO mice that was unmasked by capsaicin challenge. The heightened nociceptive response was mediated by CB1 and TRPV1 receptors and accompanied by enhanced spinal neuronal activation. Moreover, genetic deletion of FAAH has a profound impact on the peripheral and central lipidome. Thus, genetic deletion of FAAH may predispose animals to increased sensitivity to certain types of pain. More work is necessary to determine whether such changes could explain the lack of efficacy of FAAH inhibitors in clinical trials.
Keywords: FAAH knockout; Fatty-acid amide hydrolase; TRPV1; anandamide; cannabinoid CB1 receptor; capsaicin; endocannabinoid; endovanilloid.
© The Author(s) 2016.
Figures











Similar articles
-
A multi-target approach for pain treatment: dual inhibition of fatty acid amide hydrolase and TRPV1 in a rat model of osteoarthritis.Pain. 2015 May;156(5):890-903. doi: 10.1097/j.pain.0000000000000132. Pain. 2015. PMID: 25719612
-
The dual fatty acid amide hydrolase/TRPV1 blocker, N-arachidonoyl-serotonin, relieves carrageenan-induced inflammation and hyperalgesia in mice.Pharmacol Res. 2010 Jun;61(6):537-46. doi: 10.1016/j.phrs.2010.02.001. Epub 2010 Feb 6. Pharmacol Res. 2010. PMID: 20138997
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation.AAPS J. 2009 Mar;11(1):39-44. doi: 10.1208/s12248-008-9075-y. Epub 2009 Jan 29. AAPS J. 2009. PMID: 19184452 Free PMC article. Review.
-
Capsaicin, Nociception and Pain.Molecules. 2016 Jun 18;21(6):797. doi: 10.3390/molecules21060797. Molecules. 2016. PMID: 27322240 Free PMC article. Review.
Cited by
-
Mechanisms Underlining Inflammatory Pain Sensitivity in Mice Selected for High and Low Stress-Induced Analgesia-The Role of Endocannabinoids and Microglia.Int J Mol Sci. 2022 Oct 2;23(19):11686. doi: 10.3390/ijms231911686. Int J Mol Sci. 2022. PMID: 36232988 Free PMC article.
-
Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence.Biol Psychiatry. 2018 Nov 15;84(10):722-733. doi: 10.1016/j.biopsych.2017.06.032. Epub 2017 Jul 8. Biol Psychiatry. 2018. PMID: 28823711 Free PMC article.
-
Endocannabinoid-mediated potentiation of nonnociceptive synapses contributes to behavioral sensitization.J Neurophysiol. 2018 Feb 1;119(2):641-651. doi: 10.1152/jn.00092.2017. Epub 2017 Nov 8. J Neurophysiol. 2018. PMID: 29118192 Free PMC article.
-
Negative allosteric modulation of CB1 cannabinoid receptor signaling suppresses opioid-mediated tolerance and withdrawal without blocking opioid antinociception.Neuropharmacology. 2024 Oct 1;257:110052. doi: 10.1016/j.neuropharm.2024.110052. Epub 2024 Jun 25. Neuropharmacology. 2024. PMID: 38936657 Free PMC article.
-
Plasma endocannabinoids and cannabimimetic fatty acid derivatives are altered in gastroparesis: A sex- and subtype-dependent observation.Neurogastroenterol Motil. 2021 Jan;33(1):e13961. doi: 10.1111/nmo.13961. Epub 2020 Aug 10. Neurogastroenterol Motil. 2021. PMID: 32779297 Free PMC article.
References
-
- Cravatt BF, Giang DK, Mayfield SP, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996; 384: 83–87. - PubMed
-
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258: 1946–1949. - PubMed
-
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 1993 Sep 1; 46: 791–796. - PubMed
-
- Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol 2003; 7: 469–475. - PubMed
-
- Kathuria S, Gaetani S, Fegley D, et al. La modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 2002; 9: 76–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

