Interferon gamma protects neonatal neural stem/progenitor cells during measles virus infection of the brain
- PMID: 27178303
- PMCID: PMC4867982
- DOI: 10.1186/s12974-016-0571-1
Interferon gamma protects neonatal neural stem/progenitor cells during measles virus infection of the brain
Abstract
Background: In the developing brain, self-renewing neural stem/progenitor cells (NSPC) give rise to neuronal and glial lineages. NSPC survival and differentiation can be altered by neurotropic viruses and by the anti-viral immune response. Several neurotropic viruses specifically target and infect NSPCs, in addition to inducing neuronal loss, which makes it difficult to distinguish between effects on NSPCs that are due to direct viral infection or due to the anti-viral immune response.
Methods: We have investigated the impact of anti-viral immunity on NSPCs in measles virus (MV)-infected neonates. A neuron-restricted viral infection model was used, where NSPCs remain uninfected. Thus, an anti-viral immune response was induced without the confounding issue of NSPC infection. Two-transgenic mouse lines were used: CD46+ mice express the human isoform of CD46, the MV entry receptor, under the control of the neuron-specific enolase promoter; CD46+/IFNγ-KO mice lack the key anti-viral cytokine IFNγ. Multi-color flow cytometry and Western Blot analysis were used to quantify effects on NSPC, neuronal, and glial cell number, and quantify effects on IFNγ-mediated signaling and cell markers, respectively.
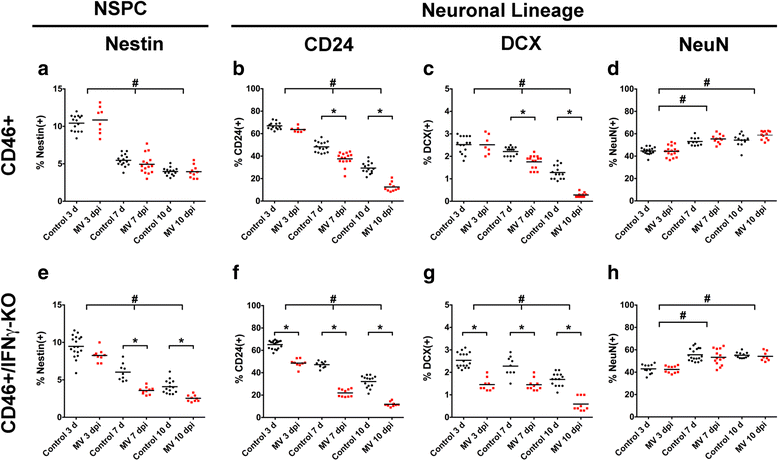
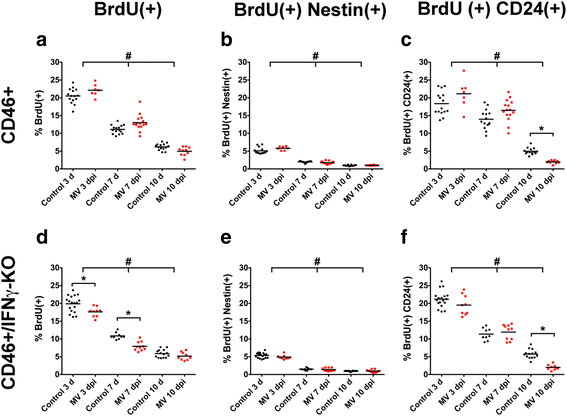
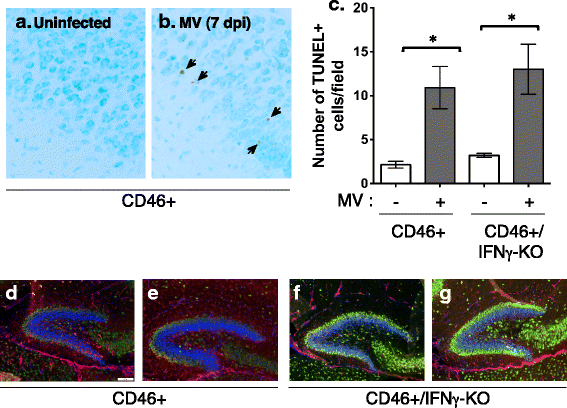
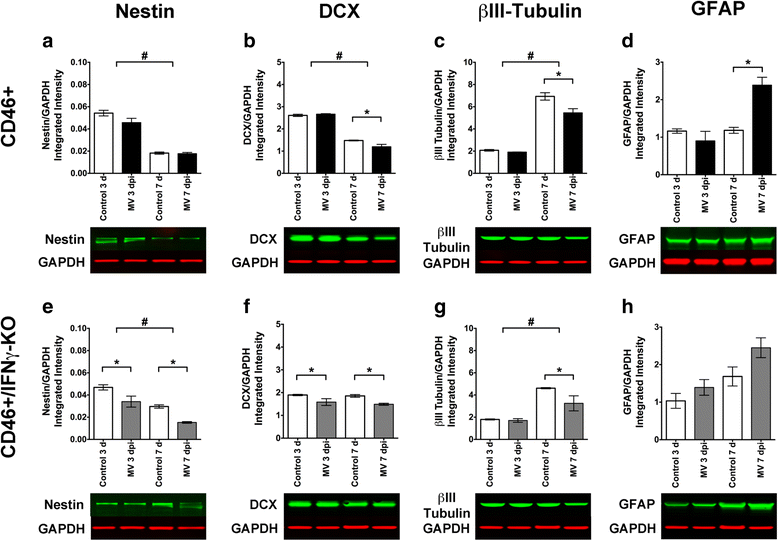
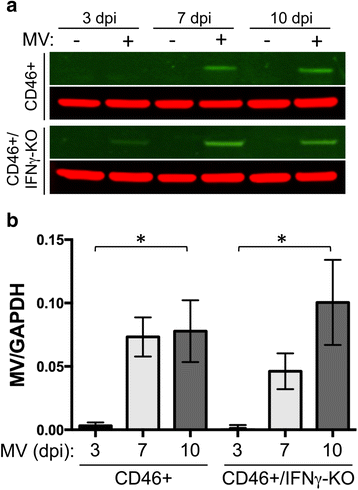
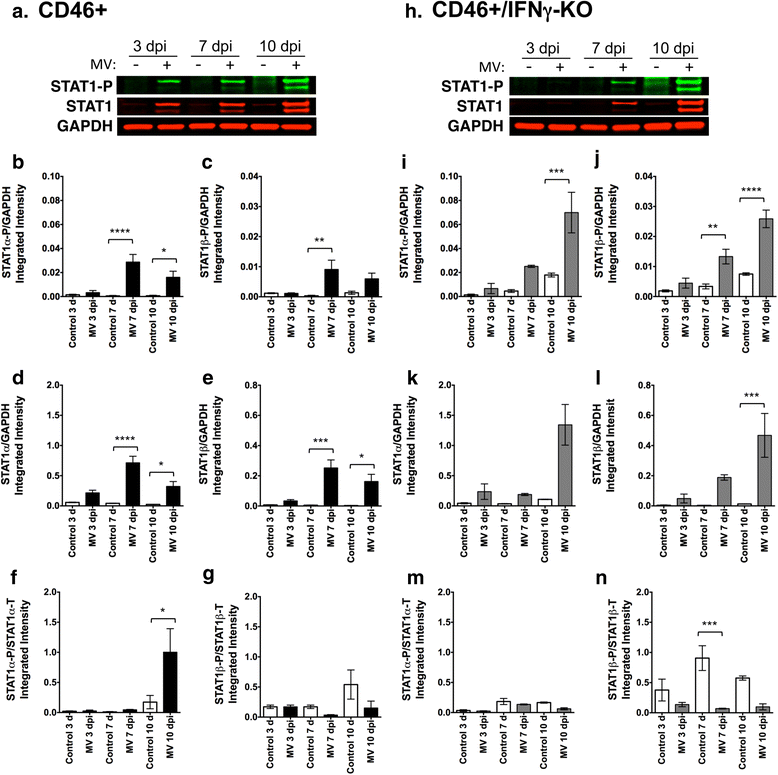
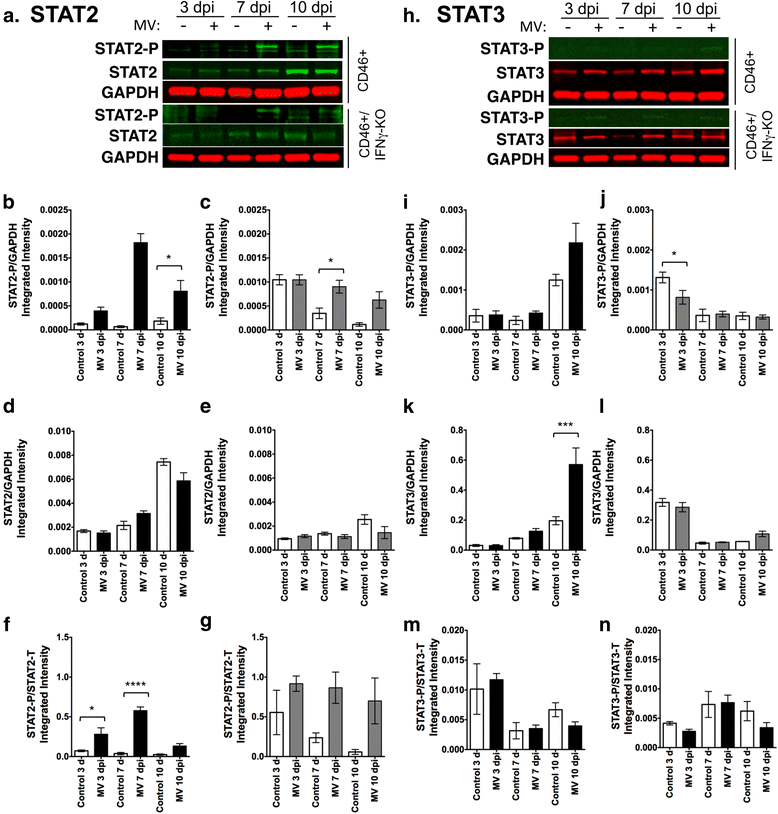
Results: Flow cytometric analysis revealed that NSPCs were reduced in CD46+/IFNγ-KO mice at 3, 7, and 10 days post-infection (dpi), but were unaffected in CD46+ mice. Early neurons showed the greatest cell loss at 7 dpi in both genotypes, with no effect on mature neurons and glial cells. Thus, IFNγ protected against NSPC loss, but did not protect young neurons. Western Blot analyses on hippocampal explants showed reduced nestin expression in the absence of IFNγ, and reduced doublecortin and βIII-tubulin in both genotypes. Phosphorylation of STAT1 and STAT2 occurred independently of IFNγ in the hippocampus, albeit with distinct regulation of activation.
Conclusions: This is the first study to demonstrate bystander effects of anti-viral immunity on NSPC function. Our results show IFNγ protects the NSPC population during a neonatal viral CNS infection. Significant loss of NSPCs in CD46+/IFNγ-KO neonates suggests that the adaptive immune response is detrimental to NSPCs in the absence of IFNγ. These results reveal the importance and contribution of the anti-viral immune response to neuropathology and may be relevant to other neuroinflammatory conditions.
Keywords: Anti-viral; Glia; Immune response; Interferon-γ; Measles virus; Neonate; Neural stem cell; Neurogenesis; STAT signaling.
Figures

Similar articles
-
Type II interferon signaling in the brain during a viral infection with age-dependent pathogenesis.Dev Neurobiol. 2020 Jul;80(7-8):213-228. doi: 10.1002/dneu.22778. Epub 2020 Sep 29. Dev Neurobiol. 2020. PMID: 32866337 Free PMC article.
-
Productive measles virus brain infection and apoptosis in CD46 transgenic mice.J Virol. 2000 Feb;74(3):1373-82. doi: 10.1128/jvi.74.3.1373-1382.2000. J Virol. 2000. PMID: 10627548 Free PMC article.
-
The anti-viral immune response of the adult host robustly modulates neural stem cell activity in spatial, temporal, and sex-specific manners.Brain Behav Immun. 2023 Nov;114:61-77. doi: 10.1016/j.bbi.2023.07.008. Epub 2023 Jul 28. Brain Behav Immun. 2023. PMID: 37516388
-
Interferon Gamma: Influence on Neural Stem Cell Function in Neurodegenerative and Neuroinflammatory Disease.Clin Med Insights Pathol. 2016 Oct 13;9(Suppl 1):9-19. doi: 10.4137/CPath.S40497. eCollection 2016. Clin Med Insights Pathol. 2016. PMID: 27774000 Free PMC article. Review.
-
Measles virus and CD46.Curr Top Microbiol Immunol. 2009;329:31-57. doi: 10.1007/978-3-540-70523-9_3. Curr Top Microbiol Immunol. 2009. PMID: 19198561 Review.
Cited by
-
Acupuncture Can Regulate the Distribution of Lymphocyte Subsets and the Levels of Inflammatory Cytokines in Patients With Mild to Moderate Vascular Dementia.Front Aging Neurosci. 2021 Nov 29;13:747673. doi: 10.3389/fnagi.2021.747673. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34912208 Free PMC article.
-
Ex vivo study of neuroinvasive and neurotropic viruses: what is current and what is next.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf024. doi: 10.1093/femsre/fuaf024. FEMS Microbiol Rev. 2025. PMID: 40498321 Free PMC article. Review.
-
MicroRNA-195 suppresses colorectal cancer cells proliferation via targeting FGF2 and regulating Wnt/β-catenin pathway.Am J Cancer Res. 2016 Nov 1;6(11):2631-2640. eCollection 2016. Am J Cancer Res. 2016. PMID: 27904776 Free PMC article.
-
Complement Has Brains-Do Intracellular Complement and Immunometabolism Cooperate in Tissue Homeostasis and Behavior?Front Immunol. 2021 Feb 25;12:629986. doi: 10.3389/fimmu.2021.629986. eCollection 2021. Front Immunol. 2021. PMID: 33717157 Free PMC article. Review.
-
Lidocaine protects H9c2 cells from hypoxia-induced injury through regulation of the MAPK/ERK/NF-κB signaling pathway.Exp Ther Med. 2019 Nov;18(5):4125-4131. doi: 10.3892/etm.2019.8055. Epub 2019 Sep 26. Exp Ther Med. 2019. PMID: 31641386 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

