Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis
- PMID: 27178468
- PMCID: PMC4991351
- DOI: 10.1016/j.immuni.2016.04.009
Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis
Abstract
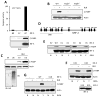
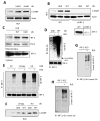
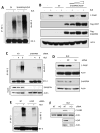
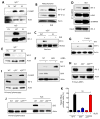
The transcription factor IRF-3 mediates cellular antiviral response by inducing the expression of interferon and other antiviral proteins. In RNA-virus infected cells, IRF-3's transcriptional activation is triggered primarily by RIG-I-like receptors (RLR), which can also activate the RLR-induced IRF-3-mediated pathway of apoptosis (RIPA). Here, we have reported that the pathway of IRF-3 activation in RIPA was independent of and distinct from the known pathway of transcriptional activation of IRF-3. It required linear polyubiquitination of two specific lysine residues of IRF-3 by LUBAC, the linear polyubiquitinating enzyme complex, which bound IRF-3 in signal-dependent fashion. To evaluate the role of RIPA in viral pathogenesis, we engineered a genetically targeted mouse, which expressed a mutant IRF-3 that was RIPA-competent but transcriptionally inert; this single-action IRF-3 could protect mice from lethal viral infection. Our observations indicated that IRF-3-mediated apoptosis of virus-infected cells could be an effective antiviral mechanism, without expression of the interferon-stimulated genes.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Belgnaoui SM, Paz S, Samuel S, Goulet ML, Sun Q, Kikkert M, Iwai K, Dikic I, Hiscott J, Lin R. Linear ubiquitination of NEMO negatively regulates the interferon antiviral response through disruption of the MAVS-TRAF3 complex. Cell host & microbe. 2012;12:211–222. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

