Insights on the virulence of swine respiratory tract mycoplasmas through genome-scale metabolic modeling
- PMID: 27178561
- PMCID: PMC4866288
- DOI: 10.1186/s12864-016-2644-z
Insights on the virulence of swine respiratory tract mycoplasmas through genome-scale metabolic modeling
Abstract
Background: The respiratory tract of swine is colonized by several bacteria among which are three Mycoplasma species: Mycoplasma flocculare, Mycoplasma hyopneumoniae and Mycoplasma hyorhinis. While colonization by M. flocculare is virtually asymptomatic, M. hyopneumoniae is the causative agent of enzootic pneumonia and M. hyorhinis is present in cases of pneumonia, polyserositis and arthritis. The genomic resemblance among these three Mycoplasma species combined with their different levels of pathogenicity is an indication that they have unknown mechanisms of virulence and differential expression, as for most mycoplasmas.
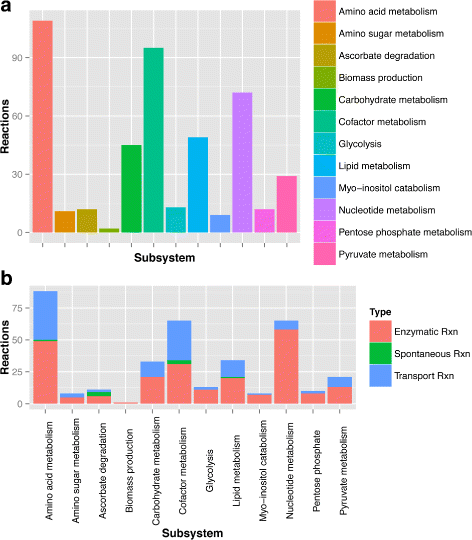
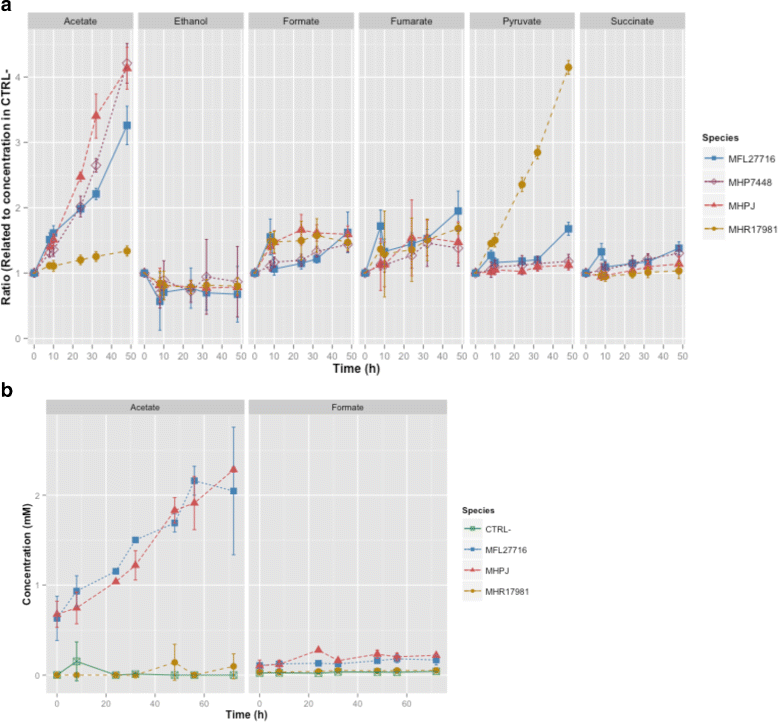
Methods: In this work, we performed whole-genome metabolic network reconstructions for these three mycoplasmas. Cultivation tests and metabolomic experiments through nuclear magnetic resonance spectroscopy (NMR) were also performed to acquire experimental data and further refine the models reconstructed in silico.
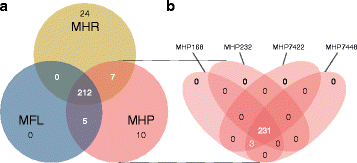
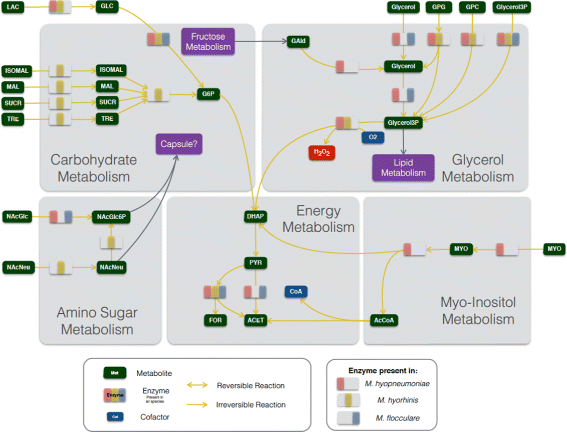
Results: Even though the refined models have similar metabolic capabilities, interesting differences include a wider range of carbohydrate uptake in M. hyorhinis, which in turn may also explain why this species is a widely contaminant in cell cultures. In addition, the myo-inositol catabolism is exclusive to M. hyopneumoniae and may be an important trait for virulence. However, the most important difference seems to be related to glycerol conversion to dihydroxyacetone-phosphate, which produces toxic hydrogen peroxide. This activity, missing only in M. flocculare, may be directly involved in cytotoxicity, as already described for two lung pathogenic mycoplasmas, namely Mycoplasma pneumoniae in human and Mycoplasma mycoides subsp. mycoides in ruminants. Metabolomic data suggest that even though these mycoplasmas are extremely similar in terms of genome and metabolism, distinct products and reaction rates may be the result of differential expression throughout the species.
Conclusions: We were able to infer from the reconstructed networks that the lack of pathogenicity of M. flocculare if compared to the highly pathogenic M. hyopneumoniae may be related to its incapacity to produce cytotoxic hydrogen peroxide. Moreover, the ability of M. hyorhinis to grow in diverse sites and even in different hosts may be a reflection of its enhanced and wider carbohydrate uptake. Altogether, the metabolic differences highlighted in silico and in vitro provide important insights to the different levels of pathogenicity observed in each of the studied species.
Keywords: Hydrogen peroxide; Metabolic network; Metabolism; Mollicutes; Mycoplasma; Whole-genome metabolic reconstruction.
Figures



References
-
- Mare CJ, Switzer WP. New species: Mycoplasma hyopneumoniae; a causative agent of virus pig pneumonia. Vet Med Small Anim Clin. 1965;60:841–6. - PubMed
-
- Rose DL, Tully JG, Wittler RG. Taxonomy of some swine mycoplasmas: Mycoplasma suipneumoniae goodwin et al. 1965, a later, objective synonym of Mycoplasma hyopneumoniae mare and switzer 1965, and the status of Mycoplasma flocculare meyling and friis 1972. Int J Syst Evol Microbiol. 1979;29(2):83–91.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

