Training primary care physicians to offer their patients faecal occult blood testing and colonoscopy for colorectal cancer screening on an equal basis: a pilot intervention with before-after and parallel group surveys
- PMID: 27178977
- PMCID: PMC4874168
- DOI: 10.1136/bmjopen-2016-011086
Training primary care physicians to offer their patients faecal occult blood testing and colonoscopy for colorectal cancer screening on an equal basis: a pilot intervention with before-after and parallel group surveys
Abstract
Objectives: Primary care physicians (PCPs) should prescribe faecal immunochemical testing (FIT) or colonoscopy for colorectal cancer screening based on their patient's values and preferences. However, there are wide variations between PCPs in the screening method prescribed. The objective was to assess the impact of an educational intervention on PCPs' intent to offer FIT or colonoscopy on an equal basis.
Design: Survey before and after training seminars, with a parallel comparison through a mailed survey to PCPs not attending the training seminars.
Setting: All PCPs in the canton of Vaud, Switzerland.
Participants: Of 592 eligible PCPs, 133 (22%) attended a seminar and 106 (80%) filled both surveys. 109 (24%) PCPs who did not attend the seminars returned the mailed survey.
Intervention: A 2 h-long interactive seminar targeting PCP knowledge, skills and attitudes regarding offering a choice of colorectal cancer (CRC) screening options.
Outcome measures: The primary outcome was PCP intention of having their patients screened with FIT and colonoscopy in equal proportions (between 40% and 60% each). Secondary outcomes were the perceived role of PCPs in screening decisions (from paternalistic to informed decision-making) and correct answer to a clinical vignette.
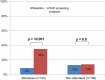
Results: Before the seminars, 8% of PCPs reported that they had equal proportions of their patients screened for CRC by FIT and colonoscopy; after the seminar, 33% foresaw having their patients screened in equal proportions (p<0.001). Among those not attending, there was no change (13% vs 14%, p=0.8). Of those attending, there was no change in their perceived role in screening decisions, while the proportion responding correctly to a clinical vignette increased (88-99%, p<0.001).
Conclusions: An interactive training seminar increased the proportion of physicians with the intention to prescribe FIT and colonoscopy in equal proportions.
Keywords: MEDICAL EDUCATION & TRAINING; PRIMARY CARE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


Similar articles
-
Screening for colorectal cancer: the role of the primary care physician.Eur J Gastroenterol Hepatol. 2017 Jan;29(1):e1-e7. doi: 10.1097/MEG.0000000000000759. Eur J Gastroenterol Hepatol. 2017. PMID: 27676092 Free PMC article. Review.
-
Variation in colorectal cancer testing between primary care physicians: a cross-sectional study in Switzerland.Int J Public Health. 2019 Sep;64(7):1075-1083. doi: 10.1007/s00038-019-01259-4. Epub 2019 Jun 15. Int J Public Health. 2019. PMID: 31201428
-
Self-reported screening practices of family physicians participating in the colorectal cancer screening program of the canton of Vaud: a cross-sectional study.BMC Fam Pract. 2020 Jun 10;21(1):103. doi: 10.1186/s12875-020-01176-z. BMC Fam Pract. 2020. PMID: 32522159 Free PMC article.
-
A physician-initiated intervention to increase colorectal cancer screening in Chinese patients.Cancer. 2018 Apr 1;124 Suppl 7(Suppl 7):1568-1575. doi: 10.1002/cncr.31287. Cancer. 2018. PMID: 29578594 Free PMC article. Clinical Trial.
-
Screening for primary aldosteronism in primary care: a scoping review.Fam Pract. 2024 Oct 8;41(5):851-856. doi: 10.1093/fampra/cmae033. Fam Pract. 2024. PMID: 38912620
Cited by
-
Screening Status as a Determinant of Choice of Colorectal Cancer Screening Method: A Population-Based Informed Survey.Gastrointest Tumors. 2021 Apr;8(2):63-70. doi: 10.1159/000512954. Epub 2021 Mar 5. Gastrointest Tumors. 2021. PMID: 33981684 Free PMC article.
-
Screening, awareness and challenges for colorectal cancer treatment in Saudi Arabia: an update.Bioinformation. 2024 Apr 30;20(4):397-403. doi: 10.6026/973206300200397. eCollection 2024. Bioinformation. 2024. PMID: 38854755 Free PMC article.
-
Screening for colorectal cancer: the role of the primary care physician.Eur J Gastroenterol Hepatol. 2017 Jan;29(1):e1-e7. doi: 10.1097/MEG.0000000000000759. Eur J Gastroenterol Hepatol. 2017. PMID: 27676092 Free PMC article. Review.
-
Physician-office vs home uptake of colorectal cancer screening using FOBT/FIT among screening-eligible US adults.Cancer Med. 2019 Dec;8(17):7408-7418. doi: 10.1002/cam4.2604. Epub 2019 Oct 21. Cancer Med. 2019. PMID: 31637870 Free PMC article.
-
Variation in colorectal cancer testing between primary care physicians: a cross-sectional study in Switzerland.Int J Public Health. 2019 Sep;64(7):1075-1083. doi: 10.1007/s00038-019-01259-4. Epub 2019 Jun 15. Int J Public Health. 2019. PMID: 31201428
References
-
- U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–37. - PubMed
-
- von Karsa L, Patnick J, Segnan N et al. , European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013;45:51–9. 10.1055/s-0032-1325997 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
