Transfer of malignant trait to BRCA1 deficient human fibroblasts following exposure to serum of cancer patients
- PMID: 27179759
- PMCID: PMC4868000
- DOI: 10.1186/s13046-016-0360-9
Transfer of malignant trait to BRCA1 deficient human fibroblasts following exposure to serum of cancer patients
Abstract
Background: It was reported that metastases might occur via transfer of biologically active blood circulating molecules from the primary tumor to distant organs rather than only migration of cancer cells. We showed in an earlier study that exposure of immortalized human embryonic kidney cells (HEK 293) to cancer patient sera, induce their transformation into undifferentiated cancers due to a horizontal transfer of malignant traits. In the present work, we tested the hypothesis that even other human cells as long as they are deficient for a single oncosuppressor gene might undergo malignant transformation when exposed to human cancer serum.
Methods: We used the CRISPR/Cas9 system to establish a stable BRCA1 knockout (KO) in human fibroblasts. The BRCA1-KO fibroblasts were exposed to cancer patients' sera or healthy patients' sera for 2 weeks. Treated cells were analyzed for cell proliferation and transformation to study their susceptibility to the oncogenic potential of cancer patients' sera and to determine the possible mechanisms underlying their hypothesized transformation.
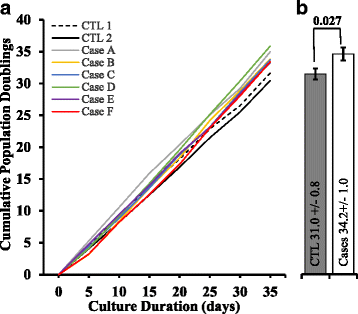
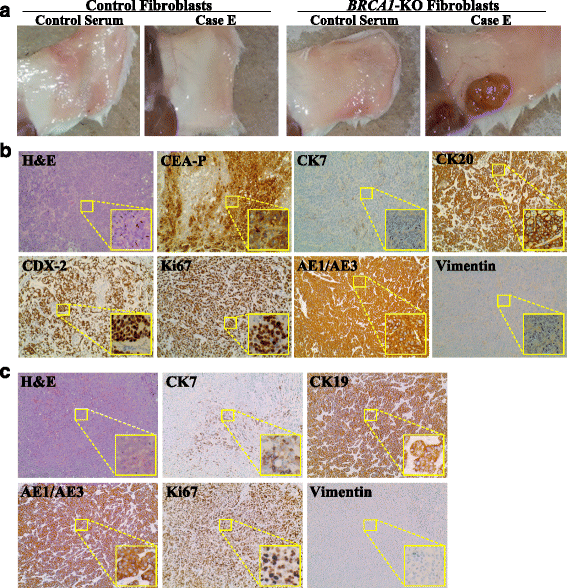
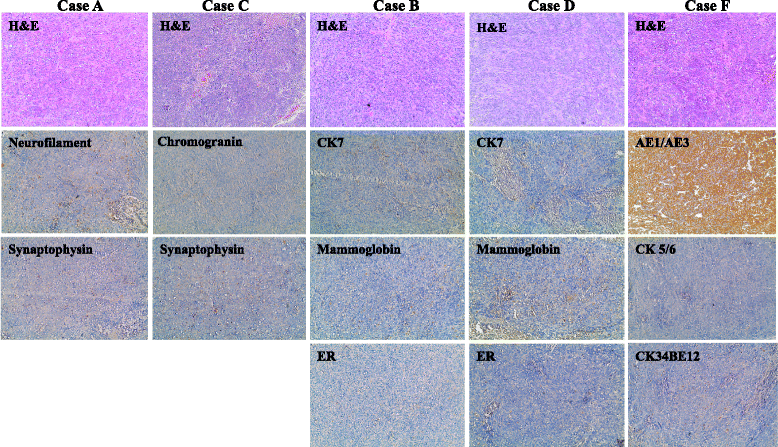
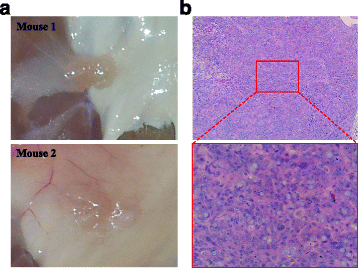
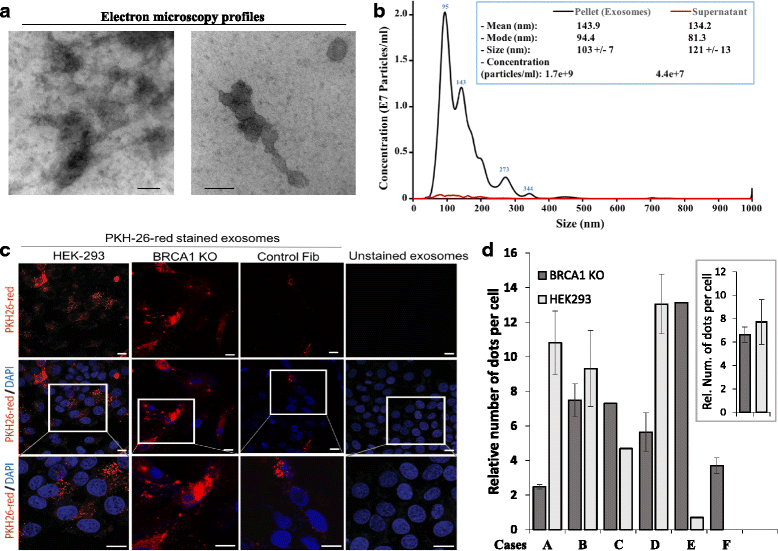
Results: BRCA1-KO fibroblasts treated with cancer patients' sera displayed higher proliferation and underwent malignant transformation as opposed to wild type control fibroblasts, which were not affected by exposure to cancer patients' sera. The malignant transformation was not seen when BRCA1-KO fibroblasts were treated with healthy human sera. Histological analysis of tumors generated by BRCA1-KO fibroblasts showed that they were carcinomas with phenotypical characteristics related to the cancers of the blood donor patients. Interestingly, BRCA1-KO fibroblasts were significantly more prone to internalize serum-derived exosomes, when compared to wild type fibroblasts. This suggests that oncosuppressor genes might protect the integrity of the cell genome also by blocking integration of cancer-derived exosomes.
Conclusion: These data support the hypothesis that any human cells carrying a single oncosuppressor mutation is capable of integrating cancer factors carried in the blood and undergo complete malignant transformation. Oncosuppressor genes might protect the cell genome by impeding the integration inside the cells of these mutating factors.
Keywords: BRCA1; Exosomes; Fibroblasts; Genometastasis; Metastasis; Transformation; Tumor suppressor genes.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

