Iron oxide nanoparticles for neuronal cell applications: uptake study and magnetic manipulations
- PMID: 27179923
- PMCID: PMC4867999
- DOI: 10.1186/s12951-016-0190-0
Iron oxide nanoparticles for neuronal cell applications: uptake study and magnetic manipulations
Abstract
Background: The ability to direct and manipulate neuronal cells has important potential in therapeutics and neural network studies. An emerging approach for remotely guiding cells is by incorporating magnetic nanoparticles (MNPs) into cells and transferring the cells into magnetic sensitive units. Recent developments offer exciting possibilities of magnetic manipulations of MNPs-loaded cells by external magnetic fields. In the present study, we evaluated and characterized uptake properties for optimal loading of cells by MNPs. We examined the interactions between MNPs of different cores and coatings, with primary neurons and neuron-like cells.
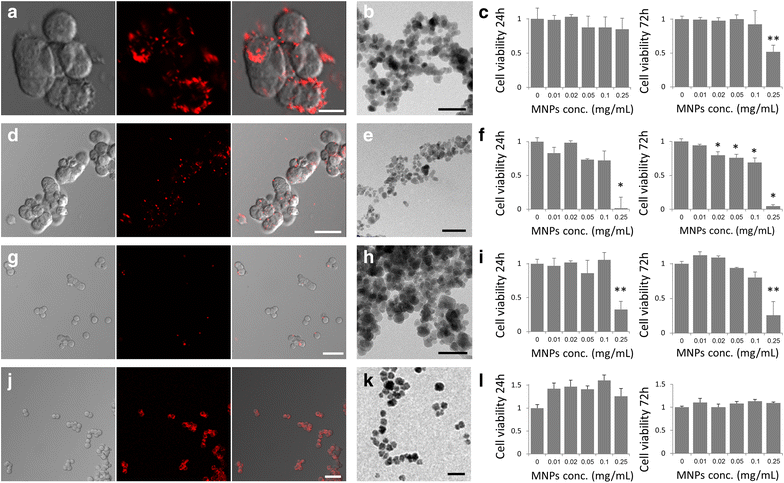
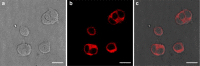
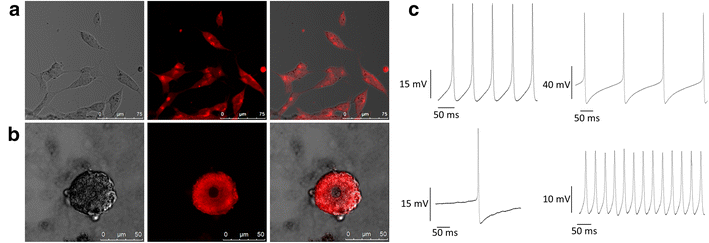
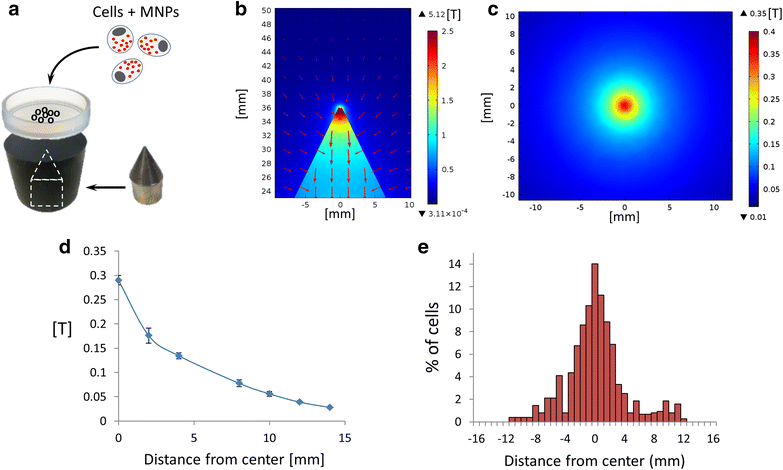
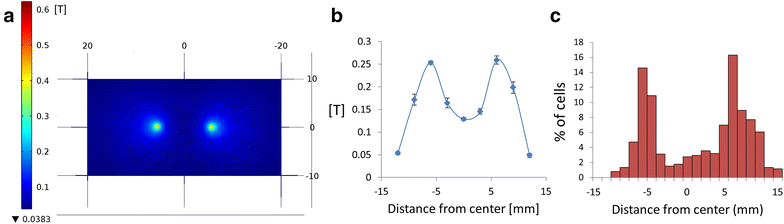
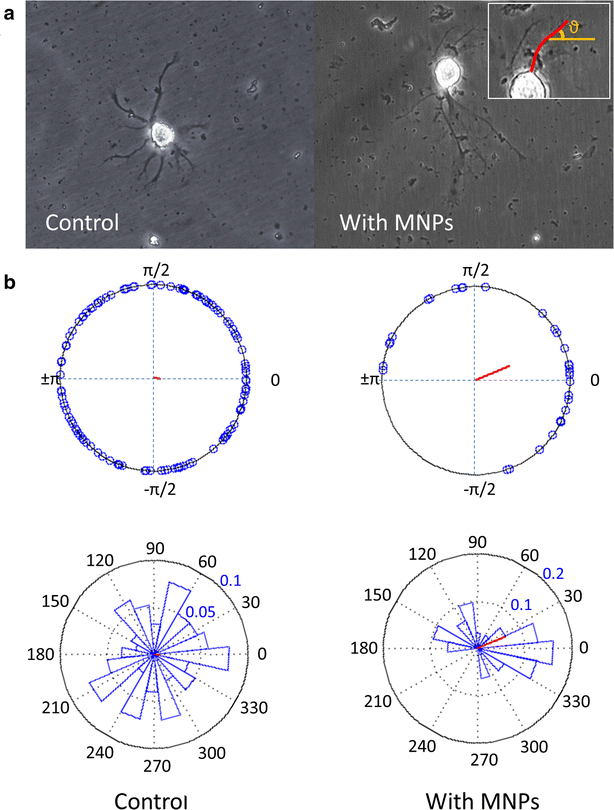
Results: We found that uncoated-maghemite iron oxide nanoparticles maximally interact and penetrate into cells with no cytotoxic effect. We observed that the cellular uptake of the MNPs depends on the time of incubation and the concentration of nanoparticles in the medium. The morphology patterns of the neuronal cells were not affected by MNPs uptake and neurons remained electrically active. We theoretically modeled magnetic fluxes and demonstrated experimentally the response of MNP-loaded cells to the magnetic fields affecting cell motility. Furthermore, we successfully directed neurite growth orientation along regeneration.
Conclusions: Applying mechanical forces via magnetic mediators is a useful approach for biomedical applications. We have examined several types of MNPs and studied the uptake behavior optimized for magnetic neuronal manipulations.
Keywords: Cell positioning; Guidance; Magnetic field; Magnetic nanoparticles; Neuronal cells; Neuronal regeneration; Uptake.
Figures


References
-
- Kilgus C, Heidsieck A, Ottersbach A, Roell W, Trueck C, Fleischmann BK, Gleich B, Sasse P. Local gene targeting and cell positioning using magnetic nanoparticles and magnetic tips: comparison of mathematical simulations with experiments. Pharm Res. 2012;29:1380–1391. doi: 10.1007/s11095-011-0647-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

