Sepsis in preterm infants causes alterations in mucosal gene expression and microbiota profiles compared to non-septic twins
- PMID: 27180802
- PMCID: PMC4867619
- DOI: 10.1038/srep25497
Sepsis in preterm infants causes alterations in mucosal gene expression and microbiota profiles compared to non-septic twins
Abstract
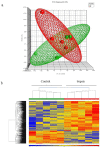
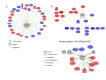
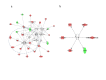
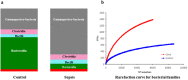
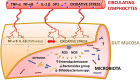
Sepsis is a life-threatening condition in preterm infants. Neonatal microbiota plays a pivotal role in the immune system maturation. Changes in gut microbiota have been associated to inflammatory disorders; however, a link with sepsis in the neonatal period has not yet been established. We aimed to analyze gut microbiota and mucosal gene expression using non-invasively obtained samples to provide with an integrative perspective of host-microbe interactions in neonatal sepsis. For this purpose, a prospective observational case-control study was conducted in septic preterm dizygotic twins and their non-septic twin controls. Fecal samples were used for both microbiota analysis and host genome-wide expression using exfoliated intestinal cells. Gene expression of exfoliated intestinal cells in septic preterm showed an induction of inflammatory and oxidative stress pathways in the gut and pro-oxidant profile that caused dysbiosis in the gut microbiota with predominance of Enterobacteria and reduction of Bacteroides and Bifidobacterium spp.in fecal samples, leading to a global reduction of beneficial anaerobic bacteria. Sepsis in preterm infants induced low-grade inflammation and oxidative stress in the gut mucosa, and also changes in the gut microbiota. This study highlights the role of inflammation and oxidative stress in neonatal sepsis on gut microbial profiles.
Figures

Similar articles
-
Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis.Pediatr Res. 2015 Jan;77(1-2):127-35. doi: 10.1038/pr.2014.161. Epub 2014 Oct 10. Pediatr Res. 2015. PMID: 25303278 Review.
-
Genome-resolved metaproteomic characterization of preterm infant gut microbiota development reveals species-specific metabolic shifts and variabilities during early life.Microbiome. 2017 Jul 10;5(1):72. doi: 10.1186/s40168-017-0290-6. Microbiome. 2017. PMID: 28693612 Free PMC article.
-
Genome-wide expression profiles in very low birth weight infants with neonatal sepsis.Pediatrics. 2014 May;133(5):e1203-11. doi: 10.1542/peds.2013-2552. Epub 2014 Apr 7. Pediatrics. 2014. PMID: 24709930
-
Gut Dysbiosis With Bacilli Dominance and Accumulation of Fermentation Products Precedes Late-onset Sepsis in Preterm Infants.Clin Infect Dis. 2019 Jul 2;69(2):268-277. doi: 10.1093/cid/ciy882. Clin Infect Dis. 2019. PMID: 30329017
-
The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants.Expert Rev Gastroenterol Hepatol. 2018 May;12(5):457-470. doi: 10.1080/17474124.2018.1446826. Epub 2018 Mar 6. Expert Rev Gastroenterol Hepatol. 2018. PMID: 29488419 Review.
Cited by
-
Microbiota and probiotics: chances and challenges - a symposium report.Gut Microbiome (Camb). 2023 Mar 27;4:e6. doi: 10.1017/gmb.2023.4. eCollection 2023. Gut Microbiome (Camb). 2023. PMID: 39295904 Free PMC article. Review.
-
The Neonatal Microbiome: Implications for Neonatal Intensive Care Unit Nurses.MCN Am J Matern Child Nurs. 2017 Nov/Dec;42(6):332-337. doi: 10.1097/NMC.0000000000000375. MCN Am J Matern Child Nurs. 2017. PMID: 29049058 Free PMC article.
-
Enteral broad-spectrum antibiotics antagonize the effect of fecal microbiota transplantation in preterm pigs.Gut Microbes. 2021 Jan-Dec;13(1):1-16. doi: 10.1080/19490976.2020.1849997. Gut Microbes. 2021. PMID: 33382952 Free PMC article.
-
Mechanisms Affecting the Gut of Preterm Infants in Enteral Feeding Trials.Front Nutr. 2017 May 8;4:14. doi: 10.3389/fnut.2017.00014. eCollection 2017. Front Nutr. 2017. PMID: 28534028 Free PMC article.
-
The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota.Microbiol Mol Biol Rev. 2017 Nov 8;81(4):e00036-17. doi: 10.1128/MMBR.00036-17. Print 2017 Dec. Microbiol Mol Biol Rev. 2017. PMID: 29118049 Free PMC article. Review.
References
-
- Zeissig S. & Blumberg R. S. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol 15, 307–10 (2014). - PubMed
-
- Cabrera-Rubio R. et al.. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 96, 544–51 (2012). - PubMed
-
- De Vos W. M. & De Vos E. A. J. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev 70, S45–S56 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

