Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS
- PMID: 27180807
- PMCID: PMC4867585
- DOI: 10.1038/srep25960
Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS
Abstract
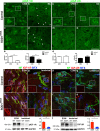
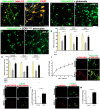
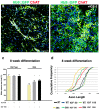
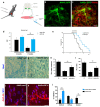
The fatal disease amyotrophic lateral sclerosis (ALS) is characterized by the loss of somatic motor neurons leading to muscle wasting and paralysis. However, motor neurons in the oculomotor nucleus, controlling eye movement, are for unknown reasons spared. We found that insulin-like growth factor 2 (IGF-2) was maintained in oculomotor neurons in ALS and thus could play a role in oculomotor resistance in this disease. We also showed that IGF-1 receptor (IGF-1R), which mediates survival pathways upon IGF binding, was highly expressed in oculomotor neurons and on extraocular muscle endplate. The addition of IGF-2 induced Akt phosphorylation, glycogen synthase kinase-3β phosphorylation and β-catenin levels while protecting ALS patient motor neurons. IGF-2 also rescued motor neurons derived from spinal muscular atrophy (SMA) patients from degeneration. Finally, AAV9::IGF-2 delivery to muscles of SOD1(G93A) ALS mice extended life-span by 10%, while preserving motor neurons and inducing motor axon regeneration. Thus, our studies demonstrate that oculomotor-specific expression can be utilized to identify candidates that protect vulnerable motor neurons from degeneration.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

