A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time
- PMID: 27180907
- PMCID: PMC4892966
- DOI: 10.1016/j.cell.2016.04.045
A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time
Erratum in
-
A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time.Cell. 2017 Jun 15;169(7):1357. doi: 10.1016/j.cell.2017.06.005. Cell. 2017. PMID: 28622515 No abstract available.
Abstract
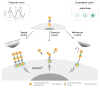
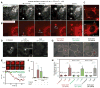
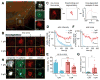
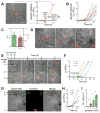
Tools capable of imaging and perturbing mechanical signaling pathways with fine spatiotemporal resolution have been elusive, despite their importance in diverse cellular processes. The challenge in developing a mechanogenetic toolkit (i.e., selective and quantitative activation of genetically encoded mechanoreceptors) stems from the fact that many mechanically activated processes are localized in space and time yet additionally require mechanical loading to become activated. To address this challenge, we synthesized magnetoplasmonic nanoparticles that can image, localize, and mechanically load targeted proteins with high spatiotemporal resolution. We demonstrate their utility by investigating the cell-surface activation of two mechanoreceptors: Notch and E-cadherin. By measuring cellular responses to a spectrum of spatial, chemical, temporal, and mechanical inputs at the single-molecule and single-cell levels, we reveal how spatial segregation and mechanical force cooperate to direct receptor activation dynamics. This generalizable technique can be used to control and understand diverse mechanosensitive processes in cell signaling. VIDEO ABSTRACT.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Small but Mighty: Nanoparticles Probe Cellular Signaling Pathways.Dev Cell. 2016 Jun 6;37(5):397-8. doi: 10.1016/j.devcel.2016.05.021. Dev Cell. 2016. PMID: 27270039 Free PMC article.
References
-
- Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW, Park SW, Shin JS, Cheon J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat Mater. 2012;11:1038–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

