Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping
- PMID: 27180912
- PMCID: PMC4873643
- DOI: 10.1038/ncomms11556
Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping
Abstract
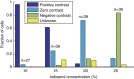
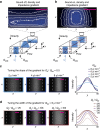
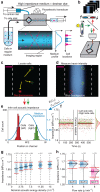
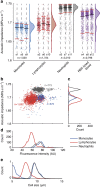
Mechanical phenotyping of single cells is an emerging tool for cell classification, enabling assessment of effective parameters relating to cells' interior molecular content and structure. Here, we present iso-acoustic focusing, an equilibrium method to analyze the effective acoustic impedance of single cells in continuous flow. While flowing through a microchannel, cells migrate sideways, influenced by an acoustic field, into streams of increasing acoustic impedance, until reaching their cell-type specific point of zero acoustic contrast. We establish an experimental procedure and provide theoretical justifications and models for iso-acoustic focusing. We describe a method for providing a suitable acoustic contrast gradient in a cell-friendly medium, and use acoustic forces to maintain that gradient in the presence of destabilizing forces. Applying this method we demonstrate iso-acoustic focusing of cell lines and leukocytes, showing that acoustic properties provide phenotypic information independent of size.
Conflict of interest statement
Although not closely related to the herein presented results P.A. declares part-time activity as a consultant in acoustofluidic research and development.
Figures

References
-
- Otto O. et al.. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199–202 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

