Melanopsin-Encoded Response Properties of Intrinsically Photosensitive Retinal Ganglion Cells
- PMID: 27181062
- PMCID: PMC4891235
- DOI: 10.1016/j.neuron.2016.04.016
Melanopsin-Encoded Response Properties of Intrinsically Photosensitive Retinal Ganglion Cells
Abstract
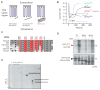
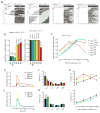
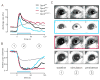
Melanopsin photopigment expressed in intrinsically photosensitive retinal ganglion cells (ipRGCs) plays a crucial role in the adaptation of mammals to their ambient light environment through both image-forming and non-image-forming visual responses. The ipRGCs are structurally and functionally distinct from classical rod/cone photoreceptors and have unique properties, including single-photon response, long response latency, photon integration over time, and slow deactivation. We discovered that amino acid sequence features of melanopsin protein contribute to the functional properties of the ipRGCs. Phosphorylation of a cluster of Ser/Thr residues in the C-terminal cytoplasmic region of melanopsin contributes to deactivation, which in turn determines response latency and threshold sensitivity of the ipRGCs. The poorly conserved region distal to the phosphorylation cluster inhibits phosphorylation's functional role, thereby constituting a unique delayed deactivation mechanism. Concerted action of both regions sustains responses to dim light, allows for the integration of light over time, and results in precise signal duration.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Melanopsin: The Tale of the Tail.Neuron. 2016 Jun 1;90(5):909-11. doi: 10.1016/j.neuron.2016.05.033. Neuron. 2016. PMID: 27253443
References
-
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. - PubMed
-
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. - PubMed
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

