Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy
- PMID: 27181261
- PMCID: PMC4931198
- DOI: 10.1111/jdi.12452
Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy
Abstract
Aims/introduction: Dental pulp stem cells (DPSCs) are thought to be an attractive candidate for cell therapy. We recently reported that the transplantation of DPSCs increased nerve conduction velocity and nerve blood flow in diabetic rats. In the present study, we investigated the immunomodulatory effects of DPSC transplantation on diabetic peripheral nerves.
Materials and methods: DPSCs were isolated from the dental pulp of Sprague-Dawley rats and expanded in culture. Eight weeks after the streptozotocin injection, DPSCs were transplanted into the unilateral hindlimb skeletal muscles. Four weeks after DPSC transplantation, neurophysiological measurements, inflammatory gene expressions and the number of CD68-positive cells in sciatic nerves were assessed. To confirm the immunomodulatory effects of DPSCs, the effects of DPSC-conditioned media on lipopolysaccharide-stimulated murine macrophage RAW264.7 cells were investigated.
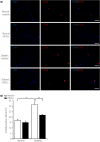
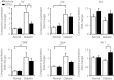
Results: Diabetic rats showed significant delays in sciatic nerve conduction velocities and decreased sciatic nerve blood flow, all of which were ameliorated by DPSC transplantation. The number of CD68-positive monocytes/macrophages and the gene expressions of M1 macrophage-expressed cytokines, tumor necrosis factor-α and interleukin-1β, were increased in the sciatic nerves of the diabetic rats. DPSC transplantation significantly decreased monocytes/macrophages and tumor necrosis factor-α messenger ribonucleic acid expression, and increased the gene expression of the M2 macrophage marker, CD206, in the sciatic nerves of the diabetic rats. The in vitro study showed that DPSC-conditioned media significantly increased the gene expressions of interleukin-10 and CD206 in lipopolysaccharide-stimulated RAW264.7 cells.
Conclusions: These results suggest that DPSC transplantation promoted macrophages polarization towards anti-inflammatory M2 phenotypes, which might be one of the therapeutic mechanisms for diabetic polyneuropathy.
Keywords: Cell transplantation; Dental pulp stem cells; Diabetic polyneuropathy.
© 2015 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures



References
-
- Naruse K, Hamada Y, Nakashima E, et al Therapeutic neovascularization using cord blood‐derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005; 54: 1823–1828. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

