Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea
- PMID: 27181367
- PMCID: PMC5148143
- DOI: 10.1164/rccm.201601-0099OC
Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea
Abstract
Rationale: Oral appliances (OAs) are commonly used as an alternative treatment to continuous positive airway pressure for patients with obstructive sleep apnea (OSA). However, OAs have variable success at reducing the apnea-hypopnea index (AHI), and predicting responders is challenging. Understanding this variability may lie with the recognition that OSA is a multifactorial disorder and that OAs may affect more than just upper-airway anatomy/collapsibility.
Objectives: The objectives of this study were to determine how OA alters AHI and four phenotypic traits (upper-airway anatomy/collapsibility and muscle function, loop gain, and arousal threshold), and baseline predictors of which patients gain the greatest benefit from therapy.
Methods: In a randomized crossover study, 14 patients with OSA attended two sleep studies with and without their OA. Under each condition, AHI and the phenotypic traits were assessed. Multiple linear regression was used to determine independent predictors of the reduction in AHI.
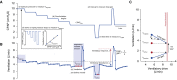
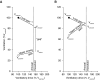
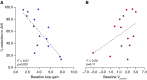
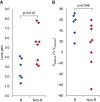
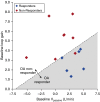
Measurements and main results: OA therapy reduced the AHI (30 ± 5 vs. 11 ± 2 events/h; P < 0.05), which was driven by improvements in upper-airway anatomy/collapsibility under passive (1.9 ± 0.7 vs. 4.7 ± 0.6 L/min; P < 0.005) and active conditions (2.4 ± 0.9 vs. 6.2 ± 0.4 L/min; P < 0.001). No changes were seen in muscle function, loop gain, or the arousal threshold. Using multivariate analysis, baseline passive upper-airway collapsibility and loop gain were independent predictors of the reduction in AHI (r2 = 0.70; P = 0.001).
Conclusions: Our findings suggest that OA therapy improves the upper-airway collapsibility under passive and active conditions. Importantly, a greater response to therapy occurred in those patients with a mild anatomic compromise and a lower loop gain.
Keywords: obstructive sleep apnea; upper-airway anatomy; ventilation.
Figures
Comment in
-
Deep Phenotyping in Obstructive Sleep Apnea. A Step Closer to Personalized Therapy.Am J Respir Crit Care Med. 2016 Dec 1;194(11):1317-1318. doi: 10.1164/rccm.201605-1003ED. Am J Respir Crit Care Med. 2016. PMID: 27905844 No abstract available.
References
-
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. - PubMed
-
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. - PubMed
-
- Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

