Single and multiple dose pharmacokinetics and pharmacodynamics of omarigliptin, a novel, once-weekly dipeptidyl peptidase-4 inhibitor, in healthy Japanese men
- PMID: 27182005
- PMCID: PMC5217871
- DOI: 10.1111/jdi.12538
Single and multiple dose pharmacokinetics and pharmacodynamics of omarigliptin, a novel, once-weekly dipeptidyl peptidase-4 inhibitor, in healthy Japanese men
Abstract
Aims/introduction: Omarigliptin is a novel, potent, long-acting oral dipeptidyl peptidase-4 inhibitor being developed as a once-weekly (q.w.) treatment for type 2 diabetes mellitus patients, with 25 mg and 12.5 mg tablets recently being approved as market formulations in Japan.
Materials and methods: This was a two-part, double-blind, randomized, placebo-controlled study in healthy Japanese men to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of omarigliptin after single dose (5-100 mg) and multiple dose (1-50 mg q.w. for 3 weeks) administration.
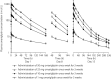
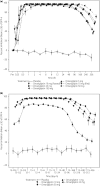
Results: Omarigliptin was rapidly absorbed with a time to maximum concentration of 0.5-4 h. The pharmacokinetic profile was biphasic with a long terminal half-life >100 h. The area under the concentration-time curve from 0 to 168 h, maximum concentration and the concentration at 168 h post-dose increased dose-dependently after 3 weeks of once-weekly dosing for doses ranging 1-50 mg, with accumulation ratios ranging 1.03-1.35 and 0.87-1.36 for the area under the concentration-time curve from 0 to 168 h and maximum concentration, respectively. Plasma dipeptidyl peptidase-4 inhibition levels 1 week post-dose increased with dose, ranging 79.2-94.0% after 5-100 mg single dose administration and 51.3-90.2% after 1-50 mg multiple once-weekly dose administration. Administration with food did not meaningfully alter the pharmacokinetics of omarigliptin. Omarigliptin was generally well tolerated, with no hypoglycemia being reported.
Conclusion: The results of the present study in healthy Japanese men showed that omarigliptin was well tolerated and had a pharmacokinetic and dipeptidyl peptidase-4 inhibition profile that supports once-weekly dosing in Japanese patients with type 2 diabetes mellitus.
Keywords: Dipeptidyl peptidase-4 inhibitors; Omarigliptin; Once-weekly.
© 2016 MSD K.K., a subsidiary of Merck & Co., Inc. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Pharmacokinetic and Pharmacodynamic Effects of Multiple-dose Administration of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor, in Obese Participants With and Without Type 2 Diabetes Mellitus.Clin Ther. 2016 Mar;38(3):516-30. doi: 10.1016/j.clinthera.2015.12.020. Epub 2016 Feb 9. Clin Ther. 2016. PMID: 26869191 Clinical Trial.
-
A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes.Diabetes Obes Metab. 2017 Nov;19(11):1602-1609. doi: 10.1111/dom.12988. Epub 2017 Jul 6. Diabetes Obes Metab. 2017. PMID: 28449368 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Pharmacodynamics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 (DPP-4) Inhibitor, After Single and Multiple Doses in Healthy Subjects.J Clin Pharmacol. 2016 Dec;56(12):1528-1537. doi: 10.1002/jcph.773. Epub 2016 Jun 17. J Clin Pharmacol. 2016. PMID: 27225334 Free PMC article. Clinical Trial.
-
[Pharmacological action and clinical results of Omarigliptin (MARIZEV® tablet), a novel dipeptidyl peptidase-4 inhibitor for once-weekly treatment of Type 2 diabetes].Nihon Yakurigaku Zasshi. 2017;149(3):128-137. doi: 10.1254/fpj.149.128. Nihon Yakurigaku Zasshi. 2017. PMID: 28260743 Review. Japanese. No abstract available.
-
Omarigliptin: first global approval.Drugs. 2015 Nov;75(16):1947-52. doi: 10.1007/s40265-015-0493-8. Drugs. 2015. PMID: 26507988 Review.
Cited by
-
Pharmacokinetics, Pharmacodynamics, and Safety of Single Dose HSK7653 Tablets in Chinese Subjects with Normal or Impaired Renal Function.Clin Pharmacokinet. 2024 Feb;63(2):227-239. doi: 10.1007/s40262-023-01333-4. Epub 2024 Jan 6. Clin Pharmacokinet. 2024. PMID: 38184489 Clinical Trial.
-
Repositioning of Omarigliptin as a once-weekly intranasal Anti-parkinsonian Agent.Sci Rep. 2018 Jun 12;8(1):8959. doi: 10.1038/s41598-018-27395-0. Sci Rep. 2018. PMID: 29895906 Free PMC article.
-
Analytical quality-by-design approach for development and validation of HPLC method for the simultaneous estimation of omarigliptin, metformin, and ezetimibe: application to human plasma and dosage forms.BMC Chem. 2023 May 5;17(1):45. doi: 10.1186/s13065-023-00955-w. BMC Chem. 2023. PMID: 37147652 Free PMC article.
-
Treatment Burden on Once-Weekly Omarigliptin Versus Daily Dipeptidyl Peptidase-4 Inhibitors in Patients with Type 2 Diabetes: Randomized Controlled Trial (ONWARD-DPP4 Study).Diabetes Ther. 2023 Oct;14(10):1639-1658. doi: 10.1007/s13300-023-01442-0. Epub 2023 Jul 19. Diabetes Ther. 2023. PMID: 37468684 Free PMC article.
-
Enhanced Extraction Technique of Omarigliptin from Human Plasma-Applied to Biological Samples from Healthy Human Volunteers.Molecules. 2020 Sep 15;25(18):4232. doi: 10.3390/molecules25184232. Molecules. 2020. PMID: 32942678 Free PMC article.
References
-
- IDF Diabetes Atlas. 7th ed Brussels, Belgium: International Diabetes Federation; http://www.diabetesatlas.org/component/attachments/
-
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004; 27: 1218–1224. - PubMed
-
- Cheong C, Barner JC, Lawson KA, et al Patient adherence and reimbursement amount for antidiabetic fixed‐dose combination products compared with dual therapy among Texas Medicaid recipients. Clin Ther 2008; 30: 1893–1907. - PubMed
-
- Paes AH, Bakker A, Soe‐Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care 1997; 20: 1512–1517. - PubMed
-
- Johnston SS, Nguyen H, Felber E, et al Retrospective study of adherence to glucagon‐like peptide‐1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther 2014; 31: 1119–1133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

