Lecithin:Retinol Acyltransferase: A Key Enzyme Involved in the Retinoid (visual) Cycle
- PMID: 27183166
- PMCID: PMC5555363
- DOI: 10.1021/acs.biochem.6b00319
Lecithin:Retinol Acyltransferase: A Key Enzyme Involved in the Retinoid (visual) Cycle
Abstract
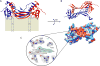
Lecithin:retinol acyltransferase (LRAT) catalyzes the acyl transfer from the sn-1 position of phosphatidylcholine (PC) to all-trans-retinol, creating fatty acid retinyl esters (palmitoyl, stearoyl, and some unsaturated derivatives). In the eye, these retinyl esters are substrates for the 65 kDa retinoid isomerase (RPE65). LRAT is well characterized biochemically, and recent structural data from closely related family members of the NlpC/P60 superfamily and a chimeric protein have established its catalytic mechanism. Mutations in the LRAT gene are responsible for approximately 1% of reported cases of Leber congenital amaurosis (LCA). Lack of functional LRAT, expressed in the retinal pigmented epithelium (RPE), results in loss of the visual chromophore and photoreceptor degeneration. LCA is a rare hereditary retinal dystrophy with an early onset associated with mutations in one of 21 known genes. Protocols have been devised to identify therapeutics that compensate for mutations in RPE65, also associated with LCA. The same protocols can be adapted to combat dystrophies associated with LRAT. Improvement in the visual function of clinical recipients of therapy with recombinant adeno-associated virus (rAAV) vectors incorporating the RPE65 gene provides a proof of concept for LRAT, which functions in the same cell type and metabolic pathway as RPE65. In parallel, a clinical trial that employs oral 9-cis-retinyl acetate to replace the missing chromophore in RPE65 and LRAT causative disease has proven to be effective and free of adverse effects. This article summarizes the biochemistry of LRAT and examines chromophore replacement as a treatment for LCA caused by LRAT mutations.
Conflict of interest statement
The authors declare the following competing financial interest(s): Treatment with 9-
Figures



References
-
- Stone EM. Leber congenital amaurosis – a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144:791–811. - PubMed
-
- Schroeder R, Mets MB, Maumenee IH. Leber’s congenital amaurosis. Retrospective review of 43 cases and a new fundus finding in two cases. Arch Ophthalmol. 1987;105:356–359. - PubMed
-
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retinal Eye Res. 2008;27:391–419. - PubMed
-
- Leber T. Uber retinitis pigmentosa und angeborene amaurose. Graefes Archiv für Ophthalmologie. 1869;15:1–25.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

