Efficacy, Safety, and Tolerability of an Extended-Release Orally Disintegrating Methylphenidate Tablet in Children 6-12 Years of Age with Attention-Deficit/Hyperactivity Disorder in the Laboratory Classroom Setting
- PMID: 27183299
- PMCID: PMC5326982
- DOI: 10.1089/cap.2016.0002
Efficacy, Safety, and Tolerability of an Extended-Release Orally Disintegrating Methylphenidate Tablet in Children 6-12 Years of Age with Attention-Deficit/Hyperactivity Disorder in the Laboratory Classroom Setting
Abstract
Objective: Methylphenidate extended-release orally disintegrating tablets (MPH XR-ODTs) represent a new technology for MPH delivery. ODTs disintegrate in the mouth without water and provide a pharmacokinetic profile that is consistent with once-daily dosing. This study sought to determine the efficacy, safety, and tolerability of this novel MPH XR-ODT formulation in school-age children with attention-deficit/hyperactivity disorder (ADHD) in a laboratory classroom setting.
Methods: Children aged 6-12 years with ADHD (n = 87) were enrolled in this randomized, multicenter, double-blind, placebo-controlled, parallel, laboratory classroom study. The MPH XR-ODT dose was titrated to an optimized dose during a 4-week open-label period and maintained on that dose for 1 week. Participants (n = 85) were then randomized to receive their optimized dose of MPH XR-ODT or placebo once daily for 1 week (double blind), culminating in a laboratory classroom testing day. Efficacy was evaluated using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Attention, Deportment, and Combined scores along with Permanent Product Measure of Performance (PERMP; Attempted and Correct) assessments. Onset and duration of drug action were also evaluated as key secondary endpoints. Safety assessments included adverse events (AEs), physical examinations, electrocardiograms (ECGs), and the Columbia Suicide Severity Rating Scale (C-SSRS).
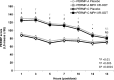
Results: The average SKAMP-Combined score on the classroom study day was significantly better for the MPH XR-ODT group (n = 43) than for the placebo group (n = 39; p < 0.0001). The effect was evident at 1 hour and lasted through 12 hours postdose. The average SKAMP-Attention, SKAMP-Deportment, PERMP-A, and PERMP-C scores were indicative of significantly greater ADHD symptom control for the MPH XR-ODT group. The most common AEs reported were decreased appetite, upper abdominal pain, headache, insomnia, upper respiratory tract infection, affect lability, irritability, cough, and vomiting.
Conclusions: MPH XR-ODT was effective and well tolerated for the treatment of children with ADHD in a laboratory classroom setting. Clinical Trial Registry: NCT01835548 ( ClinicalTrials.gov ).
Keywords: ADHD; MPH XR-ODT; classroom study; efficacy; safety.
Conflict of interest statement
Dr. A.C.C. has received research support from, consulted with, acted as an invited speaker for, and/or served on advisory boards for Alcobra Pharma, Arbor Pharmaceuticals, Forest Research Institute, Ironshore Pharmaceuticals, Lilly USA, Lundbeck, Neos Therapeutics, Neurovance, NextWave Pharmaceuticals, Noven Pharmaceuticals, Otsuka Pharmaceutical, Pfizer, Purdue Pharma, Rhodes Pharmaceuticals, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Theravance Biopharma, and Tris Pharma.
Dr. S.H.K. has received research support from and/or consulted with Akili Interactive; Alcobra Pharma; Arbor Pharmaceuticals; Atentiv; National Institutes of Health (NIDA, NIEHS, NICHD); Neos Therapeutics, Neurovance; Purdue Pharma; Rhodes Pharmaceuticals; Shire Pharmaceuticals; Sunovion Pharmaceuticals; Tris Pharma; and US Environmental Protection Agency.
Dr. A.J.C. has received research support from, consulted with, and/or acted as an invited speaker for Akili Interactive; Arbor Pharmaceuticals; AstraZeneca; Janssen Pharmaceuticals; Lilly; Lundbeck; Neos Therapeutics; Neurovance; Novartis Pharmaceuticals; Noven Pharmaceuticals; Otsuka Pharmaceutical; Pfizer; Purdue Pharma; Rhodes Pharmaceuticals; Shire Pharmaceuticals; Sunovion Pharmaceuticals; Supernus Pharmaceuticals; Takeda Pharmaceuticals; and Teva Pharmaceutical.
Dr. C.R.S. is an employee of Neos Therapeutics and has stock options. Dr. Sikes has stock in Pfizer.
Dr. A.M. has no financial relationships to disclose.
Figures


References
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV-TR). Washington, DC: American Psychiatric Association, 1994
-
- Biederman J, Faraone SV: Attention-deficit hyperactivity disorder. Lancet 366:237–248, 2005 - PubMed
-
- Chavez B, Sopko MA, Jr., Ehret MJ, Paulino RE, Goldberg KR, Angstadt K, Bogart GT: An update on central nervous system stimulant formulations in children and adolescents with attention-deficit/hyperactivity disorder. Ann Pharmacother 43:1084–1095, 2009 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

