Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae
- PMID: 27183566
- PMCID: PMC4858804
- DOI: 10.1534/genetics.115.186221
Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae
Abstract
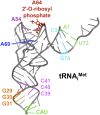
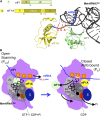
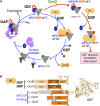
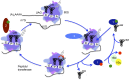
In this review, we provide an overview of protein synthesis in the yeast Saccharomyces cerevisiae The mechanism of protein synthesis is well conserved between yeast and other eukaryotes, and molecular genetic studies in budding yeast have provided critical insights into the fundamental process of translation as well as its regulation. The review focuses on the initiation and elongation phases of protein synthesis with descriptions of the roles of translation initiation and elongation factors that assist the ribosome in binding the messenger RNA (mRNA), selecting the start codon, and synthesizing the polypeptide. We also examine mechanisms of translational control highlighting the mRNA cap-binding proteins and the regulation of GCN4 and CPA1 mRNAs.
Keywords: GCN4; translation elongation; translation initiation.
Copyright © 2016 by the Genetics Society of America.
Figures





References
-
- Acker M. G., Shin B. S., Dever T. E., Lorsch J. R., 2006. Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining. J. Biol. Chem. 281: 8469–8475. - PubMed
-
- Aitken C. E., Lorsch J. R., 2012. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 19: 568–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

