Alloimmunity and Tolerance in Corneal Transplantation
- PMID: 27183635
- PMCID: PMC4874505
- DOI: 10.4049/jimmunol.1600251
Alloimmunity and Tolerance in Corneal Transplantation
Abstract
Corneal transplantation is one of the most prevalent and successful forms of solid tissue transplantation. Despite favorable outcomes, immune-mediated graft rejection remains the major cause of corneal allograft failure. Although low-risk graft recipients with uninflamed graft beds enjoy a success rate ∼90%, the rejection rates in inflamed graft beds or high-risk recipients often exceed 50%, despite maximal immune suppression. In this review, we discuss the critical facets of corneal alloimmunity, including immune and angiogenic privilege, mechanisms of allosensitization, cellular and molecular mediators of graft rejection, and allotolerance induction.
Copyright © 2016 by The American Association of Immunologists, Inc.
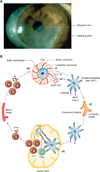
Figures


References
-
- Zirm EK. Eine erfolgreiche totale Keratoplastik (A successful total keratoplasty). 1906. Refractive & corneal surgery. 1989;5:258–261. - PubMed
-
- Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. - PubMed
-
- Eye banking statistical report. Washington, DC: Eye Bank Association of America (EBAA); 2014.
-
- Anshu A, Price MO, Price FW., Jr Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–540. - PubMed
-
- van Dooren BT, Mulder PG, Nieuwendaal CP, Beekhuis WH, Melles GR. Endothelial cell density after deep anterior lamellar keratoplasty (Melles technique) American journal of ophthalmology. 2004;137:397–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

