Stem Cells in the Intestine: Possible Roles in Pathogenesis of Irritable Bowel Syndrome
- PMID: 27184041
- PMCID: PMC4930294
- DOI: 10.5056/jnm16023
Stem Cells in the Intestine: Possible Roles in Pathogenesis of Irritable Bowel Syndrome
Abstract
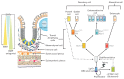
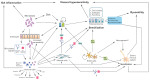
Irritable bowel syndrome is one of the most common functional gastrointestinal (GI) disorders that significantly impair quality of life in patients. Current available treatments are still not effective and the pathophysiology of this condition remains unclearly defined. Recently, research on intestinal stem cells has greatly advanced our understanding of various GI disorders. Alterations in conserved stem cell regulatory pathways such as Notch, Wnt, and bone morphogenic protein/TGF- β have been well documented in diseases such as inflammatory bowel diseases and cancer. Interaction between intestinal stem cells and various signals from their environment is important for the control of stem cell self-renewal, regulation of number and function of specific intestinal cell types, and maintenance of the mucosal barrier. Besides their roles in stem cell regulation, these signals are also known to have potent effects on immune cells, enteric nervous system and secretory cells in the gut, and may be responsible for various aspects of pathogenesis of functional GI disorders, including visceral hypersensitivity, altered gut motility and low grade gut inflammation. In this article, we briefly summarize the components of these signaling pathways, how they can be modified by extrinsic factors and novel treatments, and provide evidenced support of their roles in the inflammation processes. Furthermore, we propose how changes in these signals may contribute to the symptom development and pathogenesis of irritable bowel syndrome.
Keywords: Intestinal stem cells; Irritable bowel syndrome; Notch; Transforming growth factor beta; Wnt.
Figures


Similar articles
-
What does the future hold for irritable bowel syndrome and the functional gastrointestinal disorders?J Clin Gastroenterol. 2005 May-Jun;39(5 Suppl 3):S251-6. doi: 10.1097/01.mcg.0000156107.13247.69. J Clin Gastroenterol. 2005. PMID: 15798493 Review.
-
Gastrointestinal Physiology and Function.Handb Exp Pharmacol. 2017;239:1-16. doi: 10.1007/164_2016_118. Handb Exp Pharmacol. 2017. PMID: 28176047 Review.
-
[Stress-mast cell axis and regulation of gut mucosal inflammation: from intestinal health to an irritable bowel].Med Clin (Barc). 2007 Jun 9;129(2):61-9. doi: 10.1157/13106939. Med Clin (Barc). 2007. PMID: 17588364 Review. Spanish.
-
New insights into the pathogenesis and pathophysiology of irritable bowel syndrome.Dig Liver Dis. 2007 Mar;39(3):201-15. doi: 10.1016/j.dld.2006.10.014. Epub 2007 Jan 30. Dig Liver Dis. 2007. PMID: 17267314 Review.
-
Bacterial overgrowth as a cause of irritable bowel syndrome.Curr Opin Gastroenterol. 2006 Nov;22(6):669-73. doi: 10.1097/01.mog.0000245544.80160.46. Curr Opin Gastroenterol. 2006. PMID: 17053447 Review.
Cited by
-
Gut microbiota was modulated by moxibustion stimulation in rats with irritable bowel syndrome.Chin Med. 2018 Dec 18;13:63. doi: 10.1186/s13020-018-0220-y. eCollection 2018. Chin Med. 2018. PMID: 30574173 Free PMC article.
-
Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome.BMC Gastroenterol. 2021 Mar 5;21(1):105. doi: 10.1186/s12876-021-01693-w. BMC Gastroenterol. 2021. PMID: 33663411 Free PMC article.
-
STW 5 Herbal Preparation Modulates Wnt3a and Claudin 1 Gene Expression in Zebrafish IBS-like Model.Pharmaceuticals (Basel). 2021 Nov 28;14(12):1234. doi: 10.3390/ph14121234. Pharmaceuticals (Basel). 2021. PMID: 34959635 Free PMC article.
-
Small Maf functions in the maintenance of germline stem cells in the Drosophila testis.Redox Biol. 2018 May;15:125-134. doi: 10.1016/j.redox.2017.12.002. Epub 2017 Dec 8. Redox Biol. 2018. PMID: 29245136 Free PMC article.
-
Genetics of irritable bowel syndrome: shifting gear via biobank-scale studies.Nat Rev Gastroenterol Hepatol. 2022 Nov;19(11):689-702. doi: 10.1038/s41575-022-00662-2. Epub 2022 Aug 10. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35948782 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

