Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine
- PMID: 27184162
- PMCID: PMC4869201
- DOI: 10.1186/s12931-016-0369-9
Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine
Abstract
Background: The potential for adverse respiratory effects following exposure to electronic (e-) cigarette liquid (e-liquid) flavorings remains largely unexplored. Given the multitude of flavor permutations on the market, identification of those flavor constituents that negatively impact the respiratory tract is a daunting task. In this study we examined the impact of common e-liquid flavoring chemicals on the airway epithelium, the cellular monolayer that provides the first line of defense against inhaled particulates, pathogens, and toxicants.
Methods: We used the xCELLigence real-time cell analyzer (RTCA) as a primary high-capacity screening tool to assess cytotoxicity thresholds and physiological effects of common e-liquid flavoring chemicals on immortalized human bronchial epithelial cells (16HBE14o-). The RTCA was used secondarily to assess the capability of 16HBE14o- cells to respond to cellular signaling agonists following a 24 h exposure to select flavoring chemicals. Finally, we conducted biophysical measurements of well-differentiated primary mouse tracheal epithelial (MTE) cells with an Ussing chamber to measure the effects of e-cigarette flavoring constituents on barrier function and ion conductance.
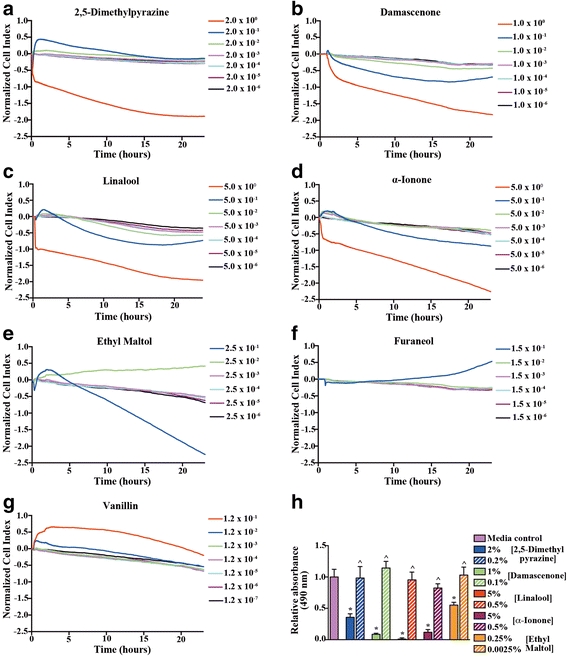
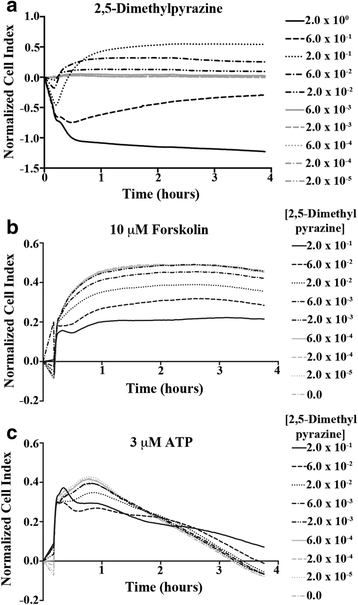
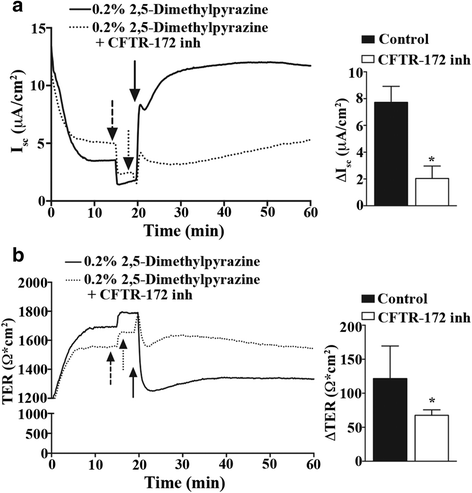
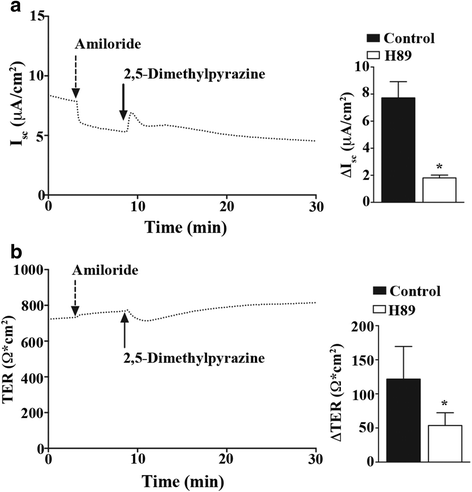
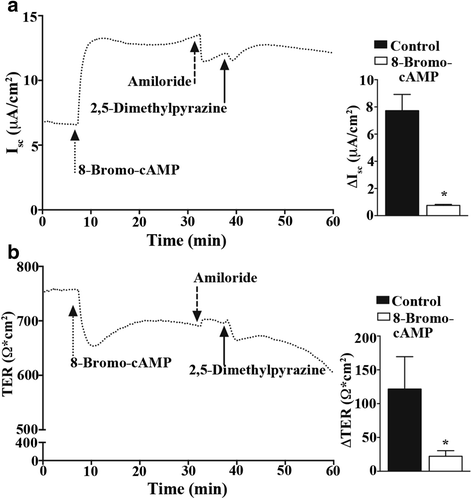
Results: In our high-capacity screens five of the seven flavoring chemicals displayed changes in cellular impedance consistent with cell death at concentrations found in e-liquid. Vanillin and the chocolate flavoring 2,5-dimethylpyrazine caused alterations in cellular physiology indicative of a cellular signaling event. At subcytotoxic levels, 24 h exposure to 2,5-dimethylpyrazine compromised the ability of airway epithelial cells to respond to signaling agonists important in salt and water balance at the airway surface. Biophysical measurements of 2,5-dimethylpyrazine on primary MTE cells revealed alterations in ion conductance consistent with an efflux at the apical airway surface that was accompanied by a transient loss in transepithelial resistance. Mechanistic studies confirmed that the increases in ion conductance evoked by 2,5-dimethylpyrazine were largely attributed to a protein kinase A-dependent (PKA) activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel.
Conclusions: Data from our high-capacity screening assays demonstrates that individual e-cigarette liquid flavoring chemicals vary in their cytotoxicity profiles and that some constituents evoke a cellular physiological response on their own independent of cell death. The activation of CFTR by 2,5-dimethylpyrazine may have detrimental consequences for airway surface liquid homeostasis in individuals that use e-cigarettes habitually.
Keywords: 2,5-dimethylpyrazine; Airway epithelium; CFTR; Electronic cigarettes; Odorant receptor; xCELLigence RTCA.
Figures

Similar articles
-
Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability.Am J Respir Cell Mol Biol. 2015 Jan;52(1):65-74. doi: 10.1165/rcmb.2013-0538OC. Am J Respir Cell Mol Biol. 2015. PMID: 24978189 Free PMC article.
-
Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia.Am J Respir Cell Mol Biol. 2019 Aug;61(2):162-173. doi: 10.1165/rcmb.2017-0432OC. Am J Respir Cell Mol Biol. 2019. PMID: 30576219 Free PMC article.
-
Cigarette smoke exposure reveals a novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR.Biochim Biophys Acta. 2015 Jun;1850(6):1224-32. doi: 10.1016/j.bbagen.2015.02.004. Epub 2015 Feb 16. Biochim Biophys Acta. 2015. PMID: 25697727 Free PMC article.
-
Electronic Cigarettes: Their Constituents and Potential Links to Asthma.Curr Allergy Asthma Rep. 2017 Oct 5;17(11):79. doi: 10.1007/s11882-017-0747-5. Curr Allergy Asthma Rep. 2017. PMID: 28983782 Free PMC article. Review.
-
Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems.Pharmacol Ther. 2021 Aug;224:107838. doi: 10.1016/j.pharmthera.2021.107838. Epub 2021 Mar 18. Pharmacol Ther. 2021. PMID: 33746051 Free PMC article. Review.
Cited by
-
An updated overview of e-cigarette impact on human health.Respir Res. 2021 May 18;22(1):151. doi: 10.1186/s12931-021-01737-5. Respir Res. 2021. PMID: 34006276 Free PMC article. Review.
-
Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients-Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors.Int J Environ Res Public Health. 2022 Dec 14;19(24):16774. doi: 10.3390/ijerph192416774. Int J Environ Res Public Health. 2022. PMID: 36554655 Free PMC article.
-
Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems.J Neurosci Methods. 2020 Jan 15;330:108458. doi: 10.1016/j.jneumeth.2019.108458. Epub 2019 Oct 12. J Neurosci Methods. 2020. PMID: 31614162 Free PMC article. Review.
-
E-Cigarettes and "Dripping" Among High-School Youth.Pediatrics. 2017 Mar;139(3):e20163224. doi: 10.1542/peds.2016-3224. Epub 2017 Feb 6. Pediatrics. 2017. PMID: 28167512 Free PMC article.
-
Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes.Drug Alcohol Depend. 2017 Sep 1;178:391-398. doi: 10.1016/j.drugalcdep.2017.05.042. Epub 2017 Jun 30. Drug Alcohol Depend. 2017. PMID: 28704768 Free PMC article.
References
-
- Johnson SR. Sparking controversy. Rise in e-cigarette use has public health experts questioning their safety, effectiveness as harm-reduction device. Mod Healthc. 2013;43:6–7. - PubMed
-
- E-cigs revolutionizing the tobacco industry. [http://www.smallcapfinancialwire.com/wp-content/uploads/2013/11/E-Cigs-R...]. Accessed 22 Sept 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

