Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide
- PMID: 27184415
- PMCID: PMC4869123
- DOI: 10.1038/srep26071
Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide
Abstract
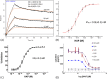
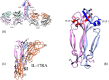
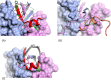
IL-17A is a pro-inflammatory cytokine that has been implicated in autoimmune and inflammatory diseases. Monoclonal antibodies inhibiting IL-17A signaling have demonstrated remarkable efficacy, but an oral therapy is still lacking. A high affinity IL-17A peptide antagonist (HAP) of 15 residues was identified through phage-display screening followed by saturation mutagenesis optimization and amino acid substitutions. HAP binds specifically to IL-17A and inhibits the interaction of the cytokine with its receptor, IL-17RA. Tested in primary human cells, HAP blocked the production of multiple inflammatory cytokines. Crystal structure studies revealed that two HAP molecules bind to one IL-17A dimer symmetrically. The N-terminal portions of HAP form a β-strand that inserts between two IL-17A monomers while the C-terminal section forms an α helix that directly blocks IL-17RA from binding to the same region of IL-17A. This mode of inhibition suggests opportunities for developing peptide antagonists against this challenging target.
Figures
Similar articles
-
Molecular modeling and rational design of disulfide-stapled self-inhibitory peptides to target IL-17A/IL-17RA interaction.J Mol Recognit. 2023 Aug;36(8):e3045. doi: 10.1002/jmr.3045. Epub 2023 Jul 6. J Mol Recognit. 2023. PMID: 37415317
-
Utilization of peptide phage display to investigate hotspots on IL-17A and what it means for drug discovery.PLoS One. 2018 Jan 12;13(1):e0190850. doi: 10.1371/journal.pone.0190850. eCollection 2018. PLoS One. 2018. PMID: 29329326 Free PMC article.
-
Crystal structures of interleukin 17A and its complex with IL-17 receptor A.Nat Commun. 2013;4:1888. doi: 10.1038/ncomms2880. Nat Commun. 2013. PMID: 23695682
-
Structural Insights into the Interleukin-17 Family Cytokines and Their Receptors.Adv Exp Med Biol. 2019;1172:97-117. doi: 10.1007/978-981-13-9367-9_5. Adv Exp Med Biol. 2019. PMID: 31628653 Review.
-
An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling.Protein Pept Lett. 2015;22(7):570-8. doi: 10.2174/0929866522666150520145554. Protein Pept Lett. 2015. PMID: 25990083 Review.
Cited by
-
Inverse behavior of IL-23R and IL-17RA in chronic and aggressive periodontitis.J Periodontal Implant Sci. 2021 Aug;51(4):254-263. doi: 10.5051/jpis.2005380269. J Periodontal Implant Sci. 2021. PMID: 34387045 Free PMC article.
-
Modulation of IL-17 backbone dynamics reduces receptor affinity and reveals a new inhibitory mechanism.Chem Sci. 2023 Jun 16;14(27):7524-7536. doi: 10.1039/d3sc00728f. eCollection 2023 Jul 12. Chem Sci. 2023. PMID: 37449080 Free PMC article.
-
Serum albumin binding knob domains engineered within a VH framework III bispecific antibody format and as chimeric peptides.Front Immunol. 2023 May 12;14:1170357. doi: 10.3389/fimmu.2023.1170357. eCollection 2023. Front Immunol. 2023. PMID: 37251411 Free PMC article.
-
Innovative peptide therapeutics targeting IL17RA to regulate inflammatory responses.Sci Rep. 2025 Mar 22;15(1):8542. doi: 10.1038/s41598-025-92915-8. Sci Rep. 2025. PMID: 40121226 Free PMC article.
-
Multicomponent Macrocyclic IL-17a Modifier.ACS Med Chem Lett. 2022 Aug 12;13(9):1468-1471. doi: 10.1021/acsmedchemlett.2c00257. eCollection 2022 Sep 8. ACS Med Chem Lett. 2022. PMID: 36105327 Free PMC article.
References
-
- Cua D. J. & Tato C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10, 479–489 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

