GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer
- PMID: 27184426
- PMCID: PMC5549011
- DOI: 10.1158/2159-8290.CD-15-0947
GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer
Abstract
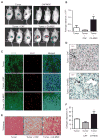
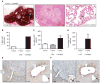
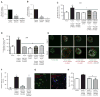
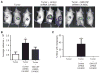
Pancreatic ductal adenocarcinoma (PDA) is characterized by a dense stroma consisting of a prevalence of activated fibroblasts whose functional contributions to pancreatic tumorigenesis remain incompletely understood. In this study, we provide the first identification and characterization of mesenchymal stem cells (MSC) within the human PDA microenvironment, highlighting the heterogeneity of the fibroblast population. Primary patient PDA samples and low-passage human pancreatic cancer-associated fibroblast cultures were found to contain a unique population of cancer-associated MSCs (CA-MSC). CA-MSCs markedly enhanced the growth, invasion, and metastatic potential of PDA cancer cells. CA-MSCs secreted the cytokine GM-CSF that was required for tumor cell proliferation, invasion, and transendothelial migration. Depletion of GM-CSF in CA-MSCs inhibited the ability of these cells to promote tumor cell growth and metastasis. Together, these data identify a population of MSCs within the tumor microenvironment that possesses a unique ability, through GM-CSF signaling, to promote PDA survival and metastasis.
Significance: The role of stroma in pancreatic cancer is controversial. Here, we provide the first characterization of MSCs within the human PDA microenvironment and demonstrate that CA-MSCs promote tumorigenesis through the production of GM-CSF. These data identify a novel cytokine pathway that mediates mesenchymal-epithelial cross-talk and is amenable to therapeutic intervention. Cancer Discov; 6(8); 886-99. ©2016 AACR.This article is highlighted in the In This Issue feature, p. 803.
©2016 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



References
-
- Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–51. 51 e1–8. - PubMed
-
- Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

