In vivo structural and cellular remodeling of engineered bone-ligament-bone constructs used for anterior cruciate ligament reconstruction in sheep
- PMID: 27184487
- PMCID: PMC5167374
- DOI: 10.1080/03008207.2016.1187141
In vivo structural and cellular remodeling of engineered bone-ligament-bone constructs used for anterior cruciate ligament reconstruction in sheep
Abstract
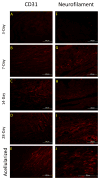
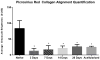
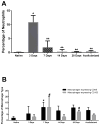
Anterior cruciate ligament (ACL) ruptures rank among the most prevalent and costly sports-related injuries. Current tendon grafts used for ACL reconstruction are limited by suboptimal biomechanical properties. We have addressed these issues by engineering multiphasic bone-ligament-bone (BLB) constructs that develop structural and mechanical properties similar to native ACL. The purpose of this study was to examine the acute remodeling process that occurs as the BLB grafts advance toward the adult ligament phenotype in vivo. Thus, we implanted BLB constructs fabricated from male cells into female host sheep and allowed 3, 7, 14, or 28 days (n = 4 at each time point) for recovery. To address whether or not graft-derived cells were even necessary, a subset of BLB constructs (n = 3) were acellularized, implanted, and allowed 28 days for recovery. At each recovery time point, the following histological analyses were performed: picrosirius red staining to assess collagen alignment and immunohistochemistry to assess both graft development and host immune response. Polymerase chain reaction (PCR) analysis, performed on every explanted BLB, was used to detect the presence of graft-derived male cells remaining in the constructs and/or migration into surrounding host tissue. The analysis of the PCR and histology samples revealed a rapid migration of host-derived macrophages and neutrophils into the graft at 3 days, followed by increased collagen density and alignment, vascularization, innervation, and near complete repopulation of the graft with host cells within 28 days. This study provides a greater understanding of the processes of ligament regeneration in our BLB constructs as they remodel toward the adult ligament phenotype.
Keywords: ACL reconstruction; remodeling; scaffoldless; tissue engineering.
Conflict of interest statement
Declaration of Interest The authors have no competing financial interests.
Figures







Similar articles
-
Allogeneic versus autologous derived cell sources for use in engineered bone-ligament-bone grafts in sheep anterior cruciate ligament repair.Tissue Eng Part A. 2015 Mar;21(5-6):1047-54. doi: 10.1089/ten.TEA.2014.0422. Epub 2015 Jan 8. Tissue Eng Part A. 2015. PMID: 25397361 Free PMC article.
-
Fresh and Frozen Tissue-Engineered Three-Dimensional Bone-Ligament-Bone Constructs for Sheep Anterior Cruciate Ligament Repair Following a 2-Year Implantation.Biores Open Access. 2016 Oct 1;5(1):289-298. doi: 10.1089/biores.2016.0032. eCollection 2016. Biores Open Access. 2016. PMID: 27843707 Free PMC article.
-
Fresh versus frozen engineered bone-ligament-bone grafts for sheep anterior cruciate ligament repair.Tissue Eng Part C Methods. 2015 Jun;21(6):548-56. doi: 10.1089/ten.TEC.2014.0542. Epub 2014 Dec 29. Tissue Eng Part C Methods. 2015. PMID: 25397990 Free PMC article.
-
Anterior Cruciate Ligament: Structure, Injuries and Regenerative Treatments.Adv Exp Med Biol. 2015;881:161-86. doi: 10.1007/978-3-319-22345-2_10. Adv Exp Med Biol. 2015. PMID: 26545750 Review.
-
Current tissue engineering strategies in anterior cruciate ligament reconstruction.J Biomed Mater Res A. 2014 May;102(5):1614-24. doi: 10.1002/jbm.a.34820. Epub 2013 Jun 14. J Biomed Mater Res A. 2014. PMID: 23737190 Review.
Cited by
-
Nerve grafting for peripheral nerve injuries with extended defect sizes.Wien Med Wochenschr. 2019 Jun;169(9-10):240-251. doi: 10.1007/s10354-018-0675-6. Epub 2018 Dec 13. Wien Med Wochenschr. 2019. PMID: 30547373 Free PMC article. Review.
-
Integration and functional performance of a decellularised porcine superflexor tendon graft in an ovine model of anterior cruciate ligament reconstruction.Biomaterials. 2021 Dec;279:121204. doi: 10.1016/j.biomaterials.2021.121204. Epub 2021 Oct 21. Biomaterials. 2021. PMID: 34736146 Free PMC article.
-
Hierarchy Reproduction: Multiphasic Strategies for Tendon/Ligament-Bone Junction Repair.Biomater Res. 2025 Jan 22;29:0132. doi: 10.34133/bmr.0132. eCollection 2025. Biomater Res. 2025. PMID: 39844867 Free PMC article. Review.
-
Engineered Tissue Graft for Repair of Injured Infraspinatus Rotator Cuff Tendon.Tissue Eng Part A. 2023 Sep;29(17-18):471-480. doi: 10.1089/ten.TEA.2022.0196. Epub 2023 Aug 31. Tissue Eng Part A. 2023. PMID: 37542392 Free PMC article.
-
Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 6-Month Recovery.Tissue Eng Part A. 2022 Jul;28(13-14):606-620. doi: 10.1089/ten.TEA.2021.0187. Tissue Eng Part A. 2022. PMID: 34937425 Free PMC article.
References
-
- Surgeons, A.A.O.S. Anterior Cruciate Ligament Injury: Surgical Considerations. 2007 Available at http://orthoinfo.aaos.org/topic.cfm?topic=A00297#A00297_R4_anchor.
-
- Fu FH, Bennett CH, Ma CB, Menetrey J, Lattermann C. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000;28(1):124–130. - PubMed
-
- Barker JU, Drakos MC, Maak TG, Warren RF, Williams RJ, Allen AA. Effect of graft selection on the incidence of postoperative infection in anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38(2):281–286. - PubMed
-
- Deacon A, Bennell K, Kiss ZS, Crossley K, Brukner P. Osteoarthritis of the knee in retired, elite Australian Rules footballers. Med J Aust. 1997;166(4):187–90. - PubMed
-
- Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
