Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury
- PMID: 27184533
- PMCID: PMC7458366
- DOI: 10.1016/j.jhep.2016.05.003
Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury
Abstract
Background & aims: Chronic outcome following acute idiosyncratic drug-induced liver injury (DILI) is not yet defined. This prospective, long-term follow-up study aimed to analyze time to liver enzyme resolutions to establish the best definition and risk factors of DILI chronicity.
Methods: 298 out of 850 patients in the Spanish DILI registry with no pre-existing disease affecting the liver and follow-up to resolution or ⩾1year were analyzed. Chronicity was defined as abnormal liver biochemistry, imaging test or histology one year after DILI recognition.
Results: Out of 298 patients enrolled 273 (92%) resolved ⩽1year from DILI recognition and 25 patients (8%) were chronic. Independent risk factors for chronicity were older age [OR: 1.06, p=0.011], dyslipidemia [OR: 4.26, p=0.04] and severe DILI [OR: 14.22, p=0.005]. Alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin (TB) median values were higher in the chronic group during follow-up. Values of ALP and TB >1.1 x upper limit of normal (xULN) and 2.8 xULN respectively, in the second month from DILI onset, were found to predict chronic DILI (p<0.001). Main drug classes involved in chronicity were statins (24%) and anti-infectives (24%). Histological examination in chronic patients demonstrated two cases with ductal lesion and seven with cirrhosis.
Conclusions: One year is the best cut-off point to define chronic DILI or prolonged recovery, with risk factors being older age, dyslipidemia and severity of the acute episode. Statins are distinctly related to chronicity. ALP and TB values in the second month could help predict chronicity or very prolonged recovery.
Lay summary: Drug-induced liver injury (DILI) patients who do not resolve their liver damage during the first year should be considered chronic DILI patients. Risk factors for DILI chronicity are older age, dyslipidemia and severity of the acute episode. Chronic DILI is not a very common condition; normally featuring mild liver profile abnormalities and not being an important clinical problem, with the exception of a small number of cases of early onset cirrhosis.
Keywords: Chronic; Hepatotoxicity; Risk factors; Statins.
Copyright © 2016 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Figures

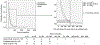
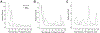
References
-
- Kaplowitz N Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 2005;4:489–499. - PubMed
-
- Lucena MI, Andrade RJ, Kaplowitz N, García-Cortes M, Fernández MC, Romero-Gomez M, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology 2009;49:2001–2009. - PubMed
-
- Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005;42:481–489. - PubMed
-
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005;129:512–521. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

