An open-label randomized control study to compare the efficacy of vitamin e versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients
- PMID: 27184636
- PMCID: PMC4898087
- DOI: 10.4103/1319-3767.182451
An open-label randomized control study to compare the efficacy of vitamin e versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients
Abstract
Background/aim: The study was carried out to compare the efficacy of Vitamin E versus Ursodeoxycholic acid (UDCA) in nondiabetic nonalcoholic fatty liver disease (NAFLD) patients.
Patients and methods: We randomized 250 non cirrhotic and non diabetic NAFLD patients diagnosed on ultrasound, with raised alanine aminotransferase (ALT) level. (>40 IU/L), to receive Vitamin E 400 mg twice a day (Group A) or UDCA 300 mg twice a day (Group B) for 52 weeks. Lifestyle modification to achieve at least 5% weight reduction and subsequent weight control and regular exercise was advised to both groups. The primary study endpoint was normalization of ALT. Secondary endpoints were the proportion of patients with reduction in ALT, relative reduction in the NAFLD Fibrosis score (NFS), symptomatic improvement and tolerability.
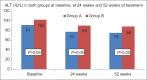
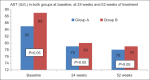
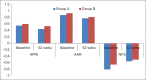
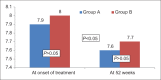
Results: One hundred and fifty patients received UDCA as compared to 100 patients receiving Vitamin E. The treatment groups were comparable at entry with regard to age (44.1 vs 42.4 years), gender (67% vs 63% female), risk factors for nonalcoholic steatohepatitis, hypochondriac pain, serum liver biochemistries, and NAFLD Fibrosis score. The primary endpoint was achieved in 21 (14%) and 19 (19%) of patients in Group A and Group B, respectively (P = 0.2). The proportion of patients with reduction in ALT (56% vs 63%, P = 0.2), symptomatic improvement (78% vs 67%, P= 0.058), reduction in the NFS (44% vs 47%, P= 0.69), and tolerability (98% vs 95%, P= 0.2) were similar between Group A and Group B, respectively.
Conclusion: UDCA is an effective and safe alternative to Vitamin E in nondiabetic-noncirrhotic Indian NAFLD patients.
Figures

Comment in
-
Is vitamin e or ursodeoxycholic acid a valid treatment option for nonalcoholic fatty liver disease in 2016?Saudi J Gastroenterol. 2016 May-Jun;22(3):169-70. doi: 10.4103/1319-3767.182462. Saudi J Gastroenterol. 2016. PMID: 27184632 Free PMC article. No abstract available.
References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. - PubMed
-
- Chitturi S, Farrell GC, George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: Future shock? J Gastroenterol Hepatol. 2004;19:368–74. - PubMed
-
- Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Asia-Pacific Working Party on NAFLD. Non-alcoholic fatty liver disease in the Asia-Pacific region: Definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–87. - PubMed
-
- Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–93. - PubMed
-
- Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
