Mild hyperglycemia triggered islet function recovery in streptozotocin-induced insulin-deficient diabetic rats
- PMID: 27184687
- PMCID: PMC5217940
- DOI: 10.1111/jdi.12540
Mild hyperglycemia triggered islet function recovery in streptozotocin-induced insulin-deficient diabetic rats
Abstract
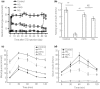
Aims/introduction: Moderate elevation of glucose level has been shown to effectively promote β-cell replication in various models in vitro and in normal rodents. Here, we aimed to test the effect of moderately elevated glucose on β-cell mass expansion and islet function recovery in diabetic animal models.
Materials and methods: A single high dose of streptozotocin was given to induce insulin-deficient diabetes in adult male Sprague-Dawley rats. Then, 48 h after streptozotocin injection, newly diabetic rats were randomly divided into three groups: (i) no treatment to maintain hyperglycemia; (ii) daily exogenous long-acting human insulin analog injection that maintained mild hyperglycemia (15 mmol/L < blood glucose < 18 mmol/L); (iii) daily exogenous long-acting human insulin analog injection to restore normoglycemia (blood glucose <8 mmol/L) as a control. Islet function, β-cell regeneration and β-cell replication were monitored during the entire analysis period.
Results: A single high dose of streptozotocin induced massive loss of β-cells, resulting in irreversible hyperglycemia. Mild hyperglycemia markedly promoted β-cell proliferation, leading to robust β-cell regeneration. Importantly, rats that maintained mild hyperglycemia showed nearly normal glucose-stimulated insulin secretion, glucose disposal and random blood glucose levels, suggesting almost full restoration of the islet function. Normalization of blood glucose levels profoundly blunted β-cell replication, regeneration and islet function recovery observed in mild hyperglycemia.
Conclusions: Our research provides a feasible approach to stimulate in situ β-cell regeneration in diabetic rats, offering new perspectives for diabetes therapy.
Keywords: Islet function recovery; Mild hyperglycemia; β-Cell proliferation.
© 2016 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures




References
-
- Hugl SR, White MF, Rhodes CJ. Insulin‐like growth factor I (IGF‐I)‐stimulated pancreatic β‐cell growth is glucose‐dependent: synergistic activation of insulin receptor substrate‐mediated signal transduction pathways by glucose and IGF‐I in INS‐1 cells. J Biol Chem 1998; 273: 17771–17779. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

