MicroRNA-93 promotes the malignant phenotypes of human glioma cells and induces their chemoresistance to temozolomide
- PMID: 27185265
- PMCID: PMC4920179
- DOI: 10.1242/bio.015552
MicroRNA-93 promotes the malignant phenotypes of human glioma cells and induces their chemoresistance to temozolomide
Abstract
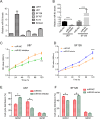
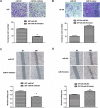
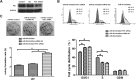
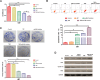
MicroRNAs (miRNAs), a class of small non-coding RNAs, can induce mRNA degradation or repress translation by binding to the 3'-untranslated region (UTR) of its target mRNA. Recently, some specific miRNAs, e.g. miR-93, have been found to be involved in pathological processes by targeting some oncogenes or tumor suppressors in glioma. However, the regulatory mechanism of miR-93 in the biological behaviors and chemoresistance of glioma cells remains unclear. In the present study, in situ hybridization and real-time RT-PCR data indicated that miR-93 was significantly upregulated in glioma patients (n=43) compared with normal brain tissues (n=8). Moreover, the upregulated miR-93 level was significantly associated with the advanced malignancy. We also found that upregulation of miR-93 promoted the proliferation, migration and invasion of glioma cells, and that miR-93 was involved in the regulation of cell cycle progression by mediating the protein levels of P21, P27, P53 and Cyclin D1. P21 was further identified as a direct target of miR-93. Knockdown of P21 attenuated the suppressive effects of miR-93 inhibition on cell cycle progression and colony formation. In addition, inhibition of miR-93 enhanced the chemosensitization of glioma cells to temozolomide (TMZ). Based on these above data, our study demonstrates that miR-93, upregulated in glioma, promotes the proliferation, cell cycle progression, migration and invasion of human glioma cells and suppresses their chemosensitivity to TMZ. Therefore, miR-93 may become a promising diagnostic marker and therapeutic target for glioma.
Keywords: Cell cycle; Glioma; Invasion; MicroRNA; Proliferation; Temozolomide.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

