Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma
- PMID: 27185615
- PMCID: PMC5016063
- DOI: 10.1634/theoncologist.2015-0478
Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma
Abstract
Background: Phase III trials show sorafenib improves survival in advanced hepatocellular carcinoma (HCC). Because of narrow trial eligibility, results may not be generalizable to a broader HCC population. We sought to evaluate the effectiveness of initial sorafenib versus no treatment among Medicare beneficiaries with advanced HCC.
Materials and methods: Patients with advanced HCC diagnosed from 2008 to 2011 were identified from the Surveillance, Epidemiology, and End Results-Medicare database. Eligible patients received initial sorafenib or no therapy and were covered by Medicare parts A, B, and D. Sorafenib use and outcomes were described in this population. Using a propensity score (PS)-matched sample, we compared the effectiveness of sorafenib versus no treatment by Cox proportional hazards and binomial regression, using a landmark requiring all patients to survive ≥60 days after diagnosis.
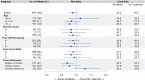
Results: Of 1,532 patients, 27% received initial sorafenib. Median duration of sorafenib use was 60 days (interquartile range [IQR], 30-107 days), and median survival from first prescription was 3 months (IQR, 1-8 months). In the PS-matched cohort, median survival was 3 months from the 60-day landmark in sorafenib-treated (n = 223) and 2 months in untreated (n = 223) patients (adjusted hazard ratio, 0.95 [95% confidence interval (CI), 0.78-1.16]). Sorafenib was associated with a nonsignificant reduction in mortality at 3 months (44% versus 51%; adjusted risk ratio, 0.88 [95% CI, 0.72-1.07]), but no reduction thereafter.
Conclusion: Survival after sorafenib initiation in newly diagnosed Medicare beneficiaries with HCC is exceptionally short, suggesting trial results are not generalizable to all HCC patients. The downsides of sorafenib use-high drug-related symptom burden and high drug cost-must be considered in light of this minimal benefit.
Implications for practice: The findings of a median survival of only 3 months in Medicare beneficiaries with HCC prescribed sorafenib as first-line therapy highlight the questionable value of sorafenib in this population. Patients should be cautioned that outside of the narrow confines of randomized trials, their life expectancy may be very short, and any benefit of sorafenib is likely to be quite small. Given that sorafenib causes considerable adverse effects and offers no symptom palliation, supportive care should be discussed as a reasonable alternative to sorafenib, particularly for patients who have a poor performance status or advanced cirrhosis.
摘要
背景. III 期临床试验显示索拉非尼改善了晚期肝细胞癌 (HCC) 患者的生存。但由于临床试验的入选标准十分狭窄, 难以适用于较广泛的 HCC 人群。我们试图在享有医保的晚期 HCC 患者中, 对初始索拉非尼治疗和不治疗的效果进行评价。
材料与方法. 从监测、流行病学和最终结果 (SEER) -医保数据库中, 识别出 2008 年至 2011 年间诊断为晚期 HCC 的患者。符合条件的患者接受初始索拉非尼治疗或不治疗均为医保 A、B 和 D 部分所覆盖。对该人群中索拉非尼的使用和转归进行描述。我们在倾向性评分 (PS) 匹配样本中, 使用所有患者诊断后生存 ≥60天作为界标, 用 Cox 比例风险和二项回归分析对索拉非尼治疗与不治疗的效果进行比较。
结果. 1 532 例患者中 27%接受了初始索拉非尼治疗。索拉非尼中位治疗时间为 60 天[四分位间距 (IQR): 30∼107天], 首次处方后的中位生存时间为 3 个月 (IQR: 1∼8个月)。在 PS 匹配队列中, 索拉非尼治疗组 (n=223) 在 60 天界标后的中位生存时间为 3 个月, 而不治疗组 (n=223) 为 2 个月[校正后风险比: 0.95, 95%置信区间 (CI): 0.78∼1.16]。索拉非尼组 3 个月死亡率低于不治疗组, 但差异无统计学意义 (44% vs. 51%, 校正后危险度: 0.88, 95%CI: 0.72∼1.07), 且之后未出现进一步下降。
结论. 享有医保的新诊断HCC患者接受初始索拉非尼治疗后的生存获益异常的短, 提示临床试验结果并不适用于所有HCC患者。考虑到索拉非尼的获益非常有限, 同时药物相关性症状负担较高, 药物花费也高, 应减少索拉非尼的使用。The Oncologist 2016;21:1113–1120
对临床实践的提示: 在享有医保的 HCC 患者中, 处方索拉非尼进行一线治疗的中位生存时间仅为 3 个月, 这对索拉非尼在这一人群中的价值提出了质疑。对于不符合随机临床试验中的狭窄限制条件的患者, 应提醒他们预期生存时间可能会非常短, 从索拉非尼治疗中得到的获益也可能相当之小。鉴于索拉非尼可引起相当多的不良事件, 而且并不能缓解症状, 应考虑将支持治疗作为合理的替代疗法, 尤其是对于体能状态不佳或合并晚期肝硬化的患者。
Keywords: Aged; Carcinoma, hepatocellular; Drug costs; Liver diseases; Liver neoplasms; Medicare; Sorafenib.
©AlphaMed Press.
Conflict of interest statement
of potential conflicts of interest may be found at the end of this article.
Figures



References
-
- Stewart BW, Wild C. eds. World cancer report 2014. International Agency for Research on Cancer . Geneva, Switzerland: WHO Press; 2014.
-
- American Cancer Society. Cancer Facts & Figures 2015. Available at http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2.... Accessed July 20, 2015.
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. - PubMed
-
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

