Crude Preparations of Helicobacter pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and mTOR-IκB Kinase-NF-κB Pathways in Dendritic Cells
- PMID: 27185786
- PMCID: PMC4962631
- DOI: 10.1128/IAI.00190-16
Crude Preparations of Helicobacter pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and mTOR-IκB Kinase-NF-κB Pathways in Dendritic Cells
Abstract
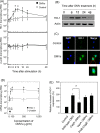
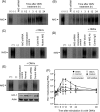
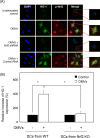
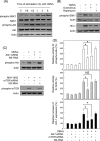
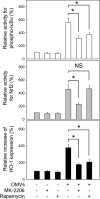
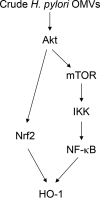
Helicobacter pylori sheds outer membrane vesicles (OMVs) that contain many surface elements of bacteria. Dendritic cells (DCs) play a major role in directing the nature of adaptive immune responses against H. pylori, and heme oxygenase-1 (HO-1) has been implicated in regulating function of DCs. In addition, HO-1 is important for adaptive immunity and the stress response. Although H. pylori-derived OMVs may contribute to the pathogenesis of H. pylori infection, responses of DCs to OMVs have not been elucidated. In the present study, we investigated the role of H. pylori-derived crude OMVs in modulating the expression of HO-1 in DCs. Exposure of DCs to crude H. pylori OMVs upregulated HO-1 expression. Crude OMVs obtained from a cagA-negative isogenic mutant strain induced less HO-1 expression than OMVs obtained from a wild-type strain. Crude H. pylori OMVs activated signals of transcription factors such as NF-κB, AP-1, and Nrf2. Suppression of NF-κB or Nrf2 resulted in significant attenuation of crude OMV-induced HO-1 expression. Crude OMVs increased the phosphorylation of Akt and downstream target molecules of mammalian target of rapamycin (mTOR), such as S6 kinase 1 (S6K1). Suppression of Akt resulted in inhibition of crude OMV-induced Nrf2-dependent HO-1 expression. Furthermore, suppression of mTOR was associated with inhibition of IκB kinase (IKK), NF-κB, and HO-1 expression in crude OMV-exposed DCs. These results suggest that H. pylori-derived OMVs regulate HO-1 expression through two different pathways in DCs, Akt-Nrf2 and mTOR-IKK-NF-κB signaling. Following this induction, increased HO-1 expression in DCs may modulate inflammatory responses in H. pylori infection.
Copyright © 2016 Ko et al.
Figures



Similar articles
-
Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases.J Biomed Sci. 2018 Nov 8;25(1):78. doi: 10.1186/s12929-018-0480-y. J Biomed Sci. 2018. PMID: 30409143 Free PMC article. Review.
-
Bacteroides fragilis enterotoxin upregulates heme oxygenase-1 in dendritic cells via reactive oxygen species-, mitogen-activated protein kinase-, and Nrf2-dependent pathway.World J Gastroenterol. 2020 Jan 21;26(3):291-306. doi: 10.3748/wjg.v26.i3.291. World J Gastroenterol. 2020. PMID: 31988590 Free PMC article.
-
Bacteroides fragilis Enterotoxin Upregulates Heme Oxygenase-1 in Intestinal Epithelial Cells via a Mitogen-Activated Protein Kinase- and NF-κB-Dependent Pathway, Leading to Modulation of Apoptosis.Infect Immun. 2016 Aug 19;84(9):2541-54. doi: 10.1128/IAI.00191-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27324483 Free PMC article.
-
Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways.J Nutr Biochem. 2013 Jan;24(1):204-12. doi: 10.1016/j.jnutbio.2012.05.003. Epub 2012 Aug 15. J Nutr Biochem. 2013. PMID: 22901690
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Mechanisms of TLR4-Mediated Autophagy and Nitroxidative Stress.Front Cell Infect Microbiol. 2021 Oct 22;11:766590. doi: 10.3389/fcimb.2021.766590. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34746034 Free PMC article. Review.
-
miR-155-regulated mTOR and Toll-like receptor 5 in gastric diffuse large B-cell lymphoma.Cancer Med. 2022 Feb;11(3):555-570. doi: 10.1002/cam4.4466. Epub 2021 Dec 16. Cancer Med. 2022. PMID: 34913612 Free PMC article.
-
Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA-Dependent Inhibition of mTORC1.Cell Host Microbe. 2018 May 9;23(5):583-593.e8. doi: 10.1016/j.chom.2018.04.006. Cell Host Microbe. 2018. PMID: 29746831 Free PMC article.
-
Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases.J Biomed Sci. 2018 Nov 8;25(1):78. doi: 10.1186/s12929-018-0480-y. J Biomed Sci. 2018. PMID: 30409143 Free PMC article. Review.
-
Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model.J Neuroinflammation. 2023 Mar 9;20(1):66. doi: 10.1186/s12974-023-02728-7. J Neuroinflammation. 2023. PMID: 36895046 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

