Iron Limitation Triggers Early Egress by the Intracellular Bacterial Pathogen Legionella pneumophila
- PMID: 27185787
- PMCID: PMC4962634
- DOI: 10.1128/IAI.01306-15
Iron Limitation Triggers Early Egress by the Intracellular Bacterial Pathogen Legionella pneumophila
Abstract
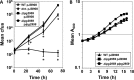
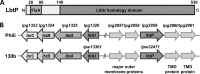
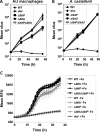
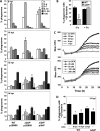
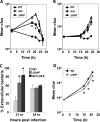
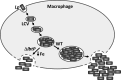
Legionella pneumophila is an intracellular bacterial pathogen that replicates in alveolar macrophages, causing a severe form of pneumonia. Intracellular growth of the bacterium depends on its ability to sequester iron from the host cell. In the L. pneumophila strain 130b, one mechanism used to acquire this essential nutrient is the siderophore legiobactin. Iron-bound legiobactin is imported by the transport protein LbtU. Here, we describe the role of LbtP, a paralog of LbtU, in iron acquisition in the L. pneumophila strain Philadelphia-1. Similar to LbtU, LbtP is a siderophore transport protein and is required for robust growth under iron-limiting conditions. Despite their similar functions, however, LbtU and LbtP do not contribute equally to iron acquisition. The Philadelphia-1 strain lacking LbtP is more sensitive to iron deprivation in vitro Moreover, LbtP is important for L. pneumophila growth within macrophages while LbtU is dispensable. These results demonstrate that LbtP plays a dominant role over LbtU in iron acquisition. In contrast, loss of both LbtP and LbtU does not impair L. pneumophila growth in the amoebal host Acanthamoeba castellanii, demonstrating a host-specific requirement for the activities of these two transporters in iron acquisition. The growth defect of the ΔlbtP mutant in macrophages is not due to alterations in growth kinetics. Instead, the absence of LbtP limits L. pneumophila replication and causes bacteria to prematurely exit the host cell. These results demonstrate the existence of a preprogrammed exit strategy in response to iron limitation that allows L. pneumophila to abandon the host cell when nutrients are exhausted.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
The Legionella pneumophila Siderophore Legiobactin Is a Polycarboxylate That Is Identical in Structure to Rhizoferrin.Infect Immun. 2015 Oct;83(10):3937-45. doi: 10.1128/IAI.00808-15. Epub 2015 Jul 20. Infect Immun. 2015. PMID: 26195554 Free PMC article.
-
lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin.J Bacteriol. 2006 Feb;188(4):1351-63. doi: 10.1128/JB.188.4.1351-1363.2006. J Bacteriol. 2006. PMID: 16452417 Free PMC article.
-
Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection.Infect Immun. 2002 Oct;70(10):5659-69. doi: 10.1128/IAI.70.10.5659-5669.2002. Infect Immun. 2002. PMID: 12228295 Free PMC article.
-
Post-translational modifications of host proteins by Legionella pneumophila: a sophisticated survival strategy.Future Microbiol. 2012 Mar;7(3):369-81. doi: 10.2217/fmb.12.9. Future Microbiol. 2012. PMID: 22393890 Review.
-
Iron acquisition by Legionella pneumophila.Biometals. 2007 Jun;20(3-4):323-31. doi: 10.1007/s10534-006-9057-4. Epub 2006 Dec 16. Biometals. 2007. PMID: 17180462 Review.
Cited by
-
Legionella pneumophila usurps host cell lipids for vacuole expansion and bacterial growth.PLoS Pathog. 2024 Feb 22;20(2):e1011996. doi: 10.1371/journal.ppat.1011996. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38386622 Free PMC article.
-
Differential Effects of Iron, Zinc, and Copper on Dictyostelium discoideum Cell Growth and Resistance to Legionella pneumophila.Front Cell Infect Microbiol. 2018 Jan 11;7:536. doi: 10.3389/fcimb.2017.00536. eCollection 2017. Front Cell Infect Microbiol. 2018. PMID: 29379774 Free PMC article.
-
Comprehensive Single Cell Analyses of the Nutritional Environment of Intracellular Salmonella enterica.Front Cell Infect Microbiol. 2021 Mar 23;11:624650. doi: 10.3389/fcimb.2021.624650. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33834004 Free PMC article.
-
Legionella pneumophila IrsA, a novel, iron-regulated exoprotein that facilitates growth in low-iron conditions and modulates biofilm formation.Microbiol Spectr. 2025 Jan 7;13(1):e0231324. doi: 10.1128/spectrum.02313-24. Epub 2024 Nov 29. Microbiol Spectr. 2025. PMID: 39612475 Free PMC article.
-
Eat or Be Eaten: Strategies Used by Legionella to Acquire Host-Derived Nutrients and Evade Lysosomal Degradation.Infect Immun. 2023 Apr 18;91(4):e0044122. doi: 10.1128/iai.00441-22. Epub 2023 Mar 13. Infect Immun. 2023. PMID: 36912646 Free PMC article. Review.
References
-
- Nguyen TM, Ilef D, Jarraud S, Rouil L, Campese C, Che D, Haieghebaert S, Ganiayre F, Marcel F, Etienne J, Desenclos JC. 2006. A community-wide outbreak of legionnaires disease linked to industrial cooling towers-how far can contaminated aerosols spread? J Infect Dis 193:102–111. doi:10.1086/498575. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

