Mammalian African trypanosome VSG coat enhances tsetse's vector competence
- PMID: 27185908
- PMCID: PMC4922192
- DOI: 10.1073/pnas.1600304113
Mammalian African trypanosome VSG coat enhances tsetse's vector competence
Abstract
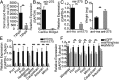
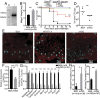
Tsetse flies are biological vectors of African trypanosomes, the protozoan parasites responsible for causing human and animal trypanosomiases across sub-Saharan Africa. Currently, no vaccines are available for disease prevention due to antigenic variation of the Variant Surface Glycoproteins (VSG) that coat parasites while they reside within mammalian hosts. As a result, interference with parasite development in the tsetse vector is being explored to reduce disease transmission. A major bottleneck to infection occurs as parasites attempt to colonize tsetse's midgut. One critical factor influencing this bottleneck is the fly's peritrophic matrix (PM), a semipermeable, chitinous barrier that lines the midgut. The mechanisms that enable trypanosomes to cross this barrier are currently unknown. Here, we determined that as parasites enter the tsetse's gut, VSG molecules released from trypanosomes are internalized by cells of the cardia-the tissue responsible for producing the PM. VSG internalization results in decreased expression of a tsetse microRNA (mir-275) and interferes with the Wnt-signaling pathway and the Iroquois/IRX transcription factor family. This interference reduces the function of the PM barrier and promotes parasite colonization of the gut early in the infection process. Manipulation of the insect midgut homeostasis by the mammalian parasite coat proteins is a novel function and indicates that VSG serves a dual role in trypanosome biology-that of facilitating transmission through its mammalian host and insect vector. We detail critical steps in the course of trypanosome infection establishment that can serve as novel targets to reduce the tsetse's vector competence and disease transmission.
Keywords: VSG; peritrophic matrix; trypanosome; tsetse; vector competence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
VSG overcomes an early barrier to survival of African trypanosomes in tsetse flies.Proc Natl Acad Sci U S A. 2016 Jun 21;113(25):6821-3. doi: 10.1073/pnas.1607008113. Epub 2016 Jun 10. Proc Natl Acad Sci U S A. 2016. PMID: 27286826 Free PMC article. No abstract available.
Comment on
-
VSG overcomes an early barrier to survival of African trypanosomes in tsetse flies.Proc Natl Acad Sci U S A. 2016 Jun 21;113(25):6821-3. doi: 10.1073/pnas.1607008113. Epub 2016 Jun 10. Proc Natl Acad Sci U S A. 2016. PMID: 27286826 Free PMC article. No abstract available.
References
-
- Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. - PubMed
-
- Shahabuddin M, Kaslow DC. Plasmodium: Parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994;79(1):85–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

