Striatal cholinergic interneurons generate beta and gamma oscillations in the corticostriatal circuit and produce motor deficits
- PMID: 27185924
- PMCID: PMC4896681
- DOI: 10.1073/pnas.1605658113
Striatal cholinergic interneurons generate beta and gamma oscillations in the corticostriatal circuit and produce motor deficits
Abstract
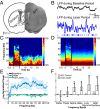
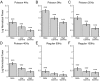
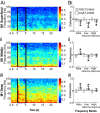
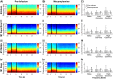
Cortico-basal ganglia-thalamic (CBT) neural circuits are critical modulators of cognitive and motor function. When compromised, these circuits contribute to neurological and psychiatric disorders, such as Parkinson's disease (PD). In PD, motor deficits correlate with the emergence of exaggerated beta frequency (15-30 Hz) oscillations throughout the CBT network. However, little is known about how specific cell types within individual CBT brain regions support the generation, propagation, and interaction of oscillatory dynamics throughout the CBT circuit or how specific oscillatory dynamics are related to motor function. Here, we investigated the role of striatal cholinergic interneurons (SChIs) in generating beta and gamma oscillations in cortical-striatal circuits and in influencing movement behavior. We found that selective stimulation of SChIs via optogenetics in normal mice robustly and reversibly amplified beta and gamma oscillations that are supported by distinct mechanisms within striatal-cortical circuits. Whereas beta oscillations are supported robustly in the striatum and all layers of primary motor cortex (M1) through a muscarinic-receptor mediated mechanism, gamma oscillations are largely restricted to the striatum and the deeper layers of M1. Finally, SChI activation led to parkinsonian-like motor deficits in otherwise normal mice. These results highlight the important role of striatal cholinergic interneurons in supporting oscillations in the CBT network that are closely related to movement and parkinsonian motor symptoms.
Keywords: beta oscillations; cholinergic interneurons; coherence; optogenetics; striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17(6):656–664. - PubMed
-
- Brown P, Williams D. Basal ganglia local field potential activity: Character and functional significance in the human. Clinical Neurophysiol. 2005;116(11):2510–2519. - PubMed
-
- Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23(7):1956–1960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

