A randomized, double-blind study comparing the efficacy and safety of a combination of formoterol and ciclesonide with ciclesonide alone in asthma subjects with moderate-to-severe airflow limitation
- PMID: 27185990
- PMCID: PMC4857562
- DOI: 10.4103/0970-2113.180803
A randomized, double-blind study comparing the efficacy and safety of a combination of formoterol and ciclesonide with ciclesonide alone in asthma subjects with moderate-to-severe airflow limitation
Abstract
Context: The combination of inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA) is widely used in the treatment of moderate-to-severe asthma uncontrolled by ICS alone.
Aims: To evaluate the efficacy and safety of a new ICS-LABA combination inhaler containing Formoterol (F) and Ciclesonide (C).
Settings and design: A double-blind, double-dummy, parallel group fashion, multi-centric study.
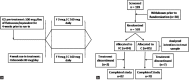
Subjects and methods: A total of 169 asthma patients received Ciclesonide 80 μg once daily during a 4-week run-in period, after which, they were randomized to receive either C (80 μg) or a combination of F (4.5 μg) and C (80 μg) (FC) both delivered through a hydro-fluro-alkane pressurized-metered-dose inhaler as 1 puff twice daily, for 6 weeks.
Statistical analysis used: Inter-group differences were compared using t-test for independent samples at a significance level of 5%.
Results: From baseline, the improvements in forced expiratory volume in 1 s at 1, 3, and 6 weeks was significantly higher in the FC group compared to Group C (110 ml vs. 40 ml, 140 ml vs. 20 ml, and 110 ml vs. 40 ml, respectively, all P < 0.05). From baseline, the improvements in mean morning peak expiratory flow at 1, 3, and 6 weeks was significantly higher in the FC group compared to Group C (17 L/min vs.-3 L/min, 22 L/min vs. 3 L/min, and 30 ml vs. 8 L/min respectively, all P < 0.05). The changes in symptom scores were similar in both the groups. The adverse events in the FC group were not significantly different from those in the C group.
Conclusions: FC provides better improvement than C alone in terms of lung function and symptoms without increased risk of adverse events in asthma patients.
Keywords: Asthma; bronchodilator agents; clinical respiratory medicine; clinical trials.
Figures


References
-
- GINAasthma.org. Global Initiative for Asthma Report 2011: Global Strategy for Asthma Management and Prevention. [Last accessed on 2012 Jul 16]. Available from: http://www.ginasthma.org/uploads/users/files/GINA_Report_2011.pdf .
-
- Todorova L, Bjermer L, Westergren-Thorsson G, Miller-Larsson A. TGFß-induced matrix production by bronchial fibroblasts in asthma: Budesonide and formoterol effects. Respir Med. 2011;105:1296–307. - PubMed
-
- Dekkers BG, Pehlic A, Mariani R, Bos IS, Meurs H, Zaagsma J. Glucocorticosteroids and ß2-adrenoceptor agonists synergize to inhibit airway smooth muscle remodeling. J Pharmacol Exp Ther. 2012;342:780–7. - PubMed
-
- Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–15. - PubMed
-
- Marceau C, Lemière C, Berbiche D, Perreault S, Blais L. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol. 2006;118:574–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

