Comparison of oral montelukast with oral zileuton in acute asthma: A randomized, double-blind, placebo-controlled study
- PMID: 27185992
- PMCID: PMC4857564
- DOI: 10.4103/0970-2113.180805
Comparison of oral montelukast with oral zileuton in acute asthma: A randomized, double-blind, placebo-controlled study
Abstract
Background: Leukotriene modifiers have an established role in the management of chronic asthma but their role in acute asthma is still under evaluation.
Objective: To study and compare the effects of oral montelukast with oral zileuton in acute asthma.
Materials and methods: This study included 120 asthmatics and was conducted from September 2012 to March 2014. Patients were randomized into three different groups to receive montelukast or zileuton or placebo in addition to standard treatment for asthma exacerbation. Peak expiratory flow rate (PEFR) values, details of rescue medication and vital signs were recorded at 6 h, 12 h, 24 h, and 48 h of drug or placebo administration and at discharge. Additional recording was done in the morning (8-10 am) following admission. The primary endpoint was the mean PEFR of each group at these time points; the secondary end point being the need for rescue medications.
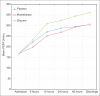
Results: The mean PEFR recordings of the three study groups - placebo, montelukast, and zileuton - respectively, at various time points were as follows: at 6 h (223.25 ± 90.40, 199.00 ± 82.52, 233.75 ± 84.05; P = 0.240); at 12 h (271.00 ± 109.38, 251.50 ± 101.44, 309.50 ± 129.63; P = 0.048); at 24 h (288.25 ± 114.26, 269.00 ± 107.51, 324.50 ± 127.88; P = 0.080); and at 48 h (295.00 ± 114.80, 293.50 ± 113.24, 344.75 ± 119.91; P = 0.015); discharge (305.00 ± 118.56, 305.25 ± 119.51, 361.25 ± 119.70; P = 0.010). The mean PEFR for the three study groups at 8-10 am on the morning following admission was 268.75 ± 111.43, 252.50 ± 99.99, 306.75 ± 114.44; P = 0.047. Total rescue doses needed were 10, 1, and 0, respectively (P = 0.049).
Conclusion: Zileuton is better than montelukast as an additional drug in acute asthma and results in significant improvement in lung function, and reduction in the need for rescue medications.
Keywords: Acute asthma; montelukast; zileuton.
Figures
References
-
- Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. - PubMed
-
- Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. - PubMed
-
- Bradshaw TA, Matusiewicz SP, Crompton GK, Innes JA, Greening AP. Intravenous magnesium sulphate provides no additive benefit to standard management in acute asthma. Respir Med. 2008;102:143–9. - PubMed
-
- Porter RS, Nester, Braitman LE, Geary U, Dalsey WC. Intravenous magnesium is ineffective in adult asthma, a randomized trial. Eur J Emerg Med. 2001;8:9–15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources