The pharmacology and therapeutic potential of (-)-huperzine A
- PMID: 27186124
- PMCID: PMC4863551
- DOI: 10.2147/JEP.S27084
The pharmacology and therapeutic potential of (-)-huperzine A
Abstract
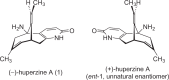
(-)-Huperzine A (1) is an alkaloid isolated from a Chinese club moss. Due to its potent neuroprotective activities, it has been investigated as a candidate for the treatment of neurodegenerative diseases, including Alzheimer's disease. In this review, we will discuss the pharmacology and therapeutic potential of (-)-huperzine A (1). Synthetic studies of (-)-huperzine A (1) aimed at enabling its development as a pharmaceutical will be described.
Keywords: Alzheimer’s; NMDA; natural products; neurodegeneration; synthesis.
Figures



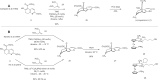

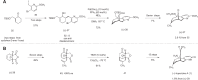
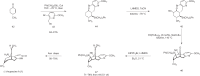
References
-
- Baladrin MF, Kinghorn AD, editors. Human Medicinal Agents from Plants: ACS Symposium Series 534. Washington, DC: American Chemical Society; 1993.
-
- Liu JS, Zhu YL, Yu CM, et al. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem. 1986;64(4):837–839.
-
- Cheng DH, Ren H, Tang XC. Huperzine A, a novel promising acetylcholinesterase inhibitor. Neuroreport. 1996;8(1):97–101. - PubMed
-
- Wang H, Tang XC. Anticholinesterase effects of huperzine A, E2020, and tacrine in rats. Zhongguo Yao Li Xue Bao. 1998;19(1):27–30. - PubMed
-
- Wang YE, Yue DX, Tang XC. Anti-cholinesterase activity of huperzine A. Zhongguo Yao Li Xue Bao. 1986;7(2):110–113. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

