Anti-tumor efficacy of BEZ235 is complemented by its anti-angiogenic effects via downregulation of PI3K-mTOR-HIF1alpha signaling in HER2-defined breast cancers
- PMID: 27186427
- PMCID: PMC4859880
Anti-tumor efficacy of BEZ235 is complemented by its anti-angiogenic effects via downregulation of PI3K-mTOR-HIF1alpha signaling in HER2-defined breast cancers
Abstract
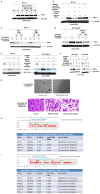
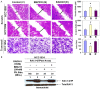
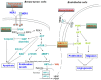
Activation of the PI3K-mTOR pathway via HER2: HER3-mediated signaling in HER2+ breast cancers pose one of the major threats towards the success of trastuzumab. First, trastuzumab cannot perturb survival/proliferative signals following HER2: HER3 heterodimerization in HER2+ tumor cells. Second, trastuzumab treatment has been reported to cause drug-mediated resistance in over 50% of HER2+ breast cancers. We have reported that treatment with an anti-angiogenic drug imparted a significant anti-tumor advantage when combined with trastuzumab plus pertuzumab in the trastuzumab-resistant model of HER2+ breast cancers (PMID: 23959459). The very fact as revealed by our study that an inclusion of anti-angiogenic drug conferred a significant anti-tumor advantage when combined with dual anti-HER2 therapy clearly indicated a critical and indispensable role of angiogenesis in these tumors. Hence, we hypothesized that BEZ235 a dual PI3K/mTOR inhibitor will have an effect on the tumor as well as the angiogenic stromal compartments. In vitro and in vivo efficacy of BEZ235 was determined in HER2+ trastuzumab-sensitive, trastuzumab-resistant and HER2 amplified/PIK3CA mutated cell lines. BEZ235 alone and in combination with trastuzumab was tested on the tumor as well as stromal compartments. AKT-mTOR signal was suppressed following BEZ235 treatment in a concentration and time-dependent manner. AnnexinV, cl-CASPASE3, SURVIVIN and p-FOXO1 indicated that BEZ235-induced cell death occurred predominantly via an apoptotic pathway. Heregulin-induced HIF1α synthesis was also significantly decreased. Oncoprint data (cBioPortal) representing PAM50 Her2 enriched tumors (TCGA, Nature 2012) and Her2-positive breast tumors (TCGA, cell 2015) showed 91.4% genetic alterations and 79.2% genetic alterations in a set of four genes comprised of PIK3CA, ERBB2, VEGFA and HIF1alpha. The co-occurrence of HIF1alpha with VEGFA in PAM50 Her2 enriched tumors (TCGA, Nature 2012) and the co-occurrence of HIF1alpha with VEGFA pair as well as HIF1alpha with PIK3CA pair in Her2-positive breast tumors (TCGA, cell 2015) were found statistically significant. In xenograft models, BEZ235 blocked tumor growth and decreased Ki67, CD31, p-AKT, p-S6RP, p-4EBP1 IHC-expressions. These decreases were more pronounced when BEZ235 was combined with trastuzumab in HER2+/trastuzumab-sensitive, trastuzumab-resistant and HER2+/PIK3CA mutated models. We demonstrated that combined targeting of HER2 and the PI3K-AKT-mTOR pathway is superior to HER2-directed therapy alone. Mechanistically the inhibition of tumor-induced angiogenesis by BEZ235 caused by the down-regulation of PI3K-mTOR-HIF1alpha signaling irrespective of the trastuzumab-sensitivity status of HER2+ breast cancers proving evidence for the first time that the inhibition of angiogenesis is an important component of the anti-tumor efficacy of BEZ235 in HER2 defined breast cancers.
Keywords: Breast cancer; HER2+; PIK3CA mutation; angiogenesis; apoptosis; trastuzumab-sensitive and trastuzumab-resistant.
Figures





References
-
- Slamon DJ, Clark GM. Amplification of cerbB-2 and aggressive human breast tumors? Science. 1988;240:1795–1798. - PubMed
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999;17:2639–2648. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
