Efficacy and safety of Vitamin D supplementation during pregnancy: A randomized trial of two different levels of dosing on maternal and neonatal Vitamin D outcome
- PMID: 27186550
- PMCID: PMC4855961
- DOI: 10.4103/2230-8210.179991
Efficacy and safety of Vitamin D supplementation during pregnancy: A randomized trial of two different levels of dosing on maternal and neonatal Vitamin D outcome
Abstract
Introduction: Pregnant women represent a typical group susceptible to dietary and mineral deficiencies. This study was sought to assess the efficacy and safety of various doses of 25-hydroxyvitamin D (25[OH]D) supplementation during pregnancy and ratify the inadequacy of the recommended daily allowance for Vitamin D in vulnerable groups.
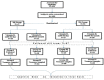
Materials and methods: A total of 100 pregnant women were included in this open-label, parallel group, prospective, randomized, and controlled trial. Study subjects were assigned to four treatment groups: Group 1 (n = 26), 1000 IU of Vitamin D daily; Group 2 (n = 21), 30,000 IU of Vitamin D monthly; Group 3 (n = 27), 2000 IU of Vitamin D daily; and Group 4 (n = 26), 60,000 IU Vitamin D monthly. Group 1 and 2 were further analyzed together as Group 1K (1000 IU daily and 30,000 IU monthly), and Group 3 and 4 as Group 2K (2000 IU daily and 60,000 IU monthly). The analysis was done on an intention to treat basis.
Results: A total of 87 patients completed the study; 21 in Group 1, 25 in Group 2, 18 in Group 3, and 23 in Group 4. The levels of 25(OH)D at baseline ranged from 1.3 to 58.0 with a mean of 24.2 ± 15.1 ng/ml. Postsupplementation, 25(OH)D levels ranged from 11.5 to 70.3 with a mean of 40.2 ± 12.2 ng/ml. The postsupplementation levels of 25(OH)D were higher in Group 2K (42.86 ± 12.83) than in Group 1K (36.96 ± 10.56) with P value of 0.023.
Conclusion: We concluded that Vitamin D supplementation with 2000 IU/day or 60,000 IU/month is very effective and safe in achieving Vitamin D sufficiency in pregnant women.
Keywords: Dose; Vitamin D; efficacy; pregnancy.
Figures
References
-
- Kaludjerovic J, Vieth R. Relationship between Vitamin D during perinatal development and health. J Midwifery Womens Health. 2010;55:550–60. - PubMed
-
- Salle BL, Delvin E, Glorieux F. Vitamin D and pregnancy. Bull Acad Natl Med. 2002;186:369–76. - PubMed
-
- Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in Northern India. Clin Endocrinol (Oxf) 2009;70:680–4. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources