Implementation of Xpert MTB/RIF in Uganda: Missed Opportunities to Improve Diagnosis of Tuberculosis
- PMID: 27186589
- PMCID: PMC4866550
- DOI: 10.1093/ofid/ofw068
Implementation of Xpert MTB/RIF in Uganda: Missed Opportunities to Improve Diagnosis of Tuberculosis
Abstract
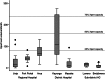
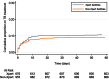
Background. The effect of Xpert MTB/RIF (Xpert) scale-up on patient outcomes in low-income settings with a high tuberculosis (TB) burden has not been established. We sought to characterize the effectiveness of Xpert as implemented across different levels of the healthcare system in Uganda. Methods. We reviewed laboratory records from 2012 to 2014 at 18 health facilities throughout Uganda. In 8 facilities, Xpert had been implemented onsite since 2012, and in 10 sites Xpert was available as an offsite referral test from another facility. We describe Xpert testing volumes by facility, Xpert and smear microscopy results, and downtime due to malfunction and cartridge stockouts. We compare TB treatment initiation as well as time to treatment between facilities implementing Xpert and those that did not. Results. The median number of Xpert assays run at implementing facilities was 25/month (interquartile range [IQR], 10-63), amounting to 8% of total capacity. Among 1251 assays run for a new TB diagnosis, 19% were positive. Among 1899 patients with smear-negative presumptive TB, the proportion starting TB treatment was similar between Xpert facilities (11%; 95% confidence interval [CI], 9%-13%) and non-Xpert facilities (9%; 95% CI, 8%-11%; P = .325). In Xpert facilities, a positive Xpert preceded TB treatment initiation in only 12 of 70 (17%) smear-negative patients initiated on treatment. Conclusions. Xpert was underutilized in Uganda and did not significantly increase the number of patients starting treatment for TB. Greater attention must be paid to appropriate implementation of novel diagnostic tests for TB if these new tools are to impact patient important outcomes.
Keywords: Uganda; Xpert MTB/RIF; implementation science; tuberculosis.
Figures

References
-
- World Health Organization. A Review of Current Epidemiological Data and Estimation of Future Tuberculosis Incidence and Mortality. Geneva; World Health Organization; 1993.
-
- World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; World Health Organization; 2015.
-
- World Health Organization. Global Strategy and Targets for Tuberculosis Prevention, Care and Control After 2015. Geneva, Switzerland; World Health Organization; 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

