Activation of RhoA, but Not Rac1, Mediates Early Stages of S1P-Induced Endothelial Barrier Enhancement
- PMID: 27187066
- PMCID: PMC4871357
- DOI: 10.1371/journal.pone.0155490
Activation of RhoA, but Not Rac1, Mediates Early Stages of S1P-Induced Endothelial Barrier Enhancement
Abstract
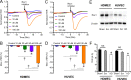
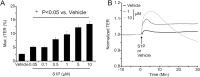
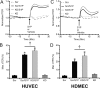
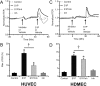
Compromised endothelial barrier function is a hallmark of inflammation. Rho family GTPases are critical in regulating endothelial barrier function, yet their precise roles, particularly in sphingosine-1-phosphate (S1P)-induced endothelial barrier enhancement, remain elusive. Confluent cultures of human umbilical vein endothelial cells (HUVEC) or human dermal microvascular endothelial cells (HDMEC) were used to model the endothelial barrier. Barrier function was assessed by determining the transendothelial electrical resistance (TER) using an electrical cell-substrate impedance sensor (ECIS). The roles of Rac1 and RhoA were tested in S1P-induced barrier enhancement. The results show that pharmacologic inhibition of Rac1 with Z62954982 failed to block S1P-induced barrier enhancement. Likewise, expression of a dominant negative form of Rac1, or knockdown of native Rac1 with siRNA, failed to block S1P-induced elevations in TER. In contrast, blockade of RhoA with the combination of the inhibitors Rhosin and Y16 significantly reduced S1P-induced increases in TER. Assessment of RhoA activation in real time using a fluorescence resonance energy transfer (FRET) biosensor showed that S1P increased RhoA activation primarily at the edges of cells, near junctions. This was complemented by myosin light chain-2 phosphorylation at cell edges, and increased F-actin and vinculin near intercellular junctions, which could all be blocked with pharmacologic inhibition of RhoA. The results suggest that S1P causes activation of RhoA at the cell periphery, stimulating local activation of the actin cytoskeleton and focal adhesions, and resulting in endothelial barrier enhancement. S1P-induced Rac1 activation, however, does not appear to have a significant role in this process.
Conflict of interest statement
Figures





References
-
- Olanders K, Sun Z, Borjesson A, Dib M, Andersson E, Lasson A, et al. (2002) The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock 18: 86–92. - PubMed
-
- Eltzschig HK, Collard CD (2004) Vascular ischaemia and reperfusion injury. Br Med Bull 70: 71–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

