Inhibitors of the Hydrolytic Enzyme Dimethylarginine Dimethylaminohydrolase (DDAH): Discovery, Synthesis and Development
- PMID: 27187323
- PMCID: PMC6273216
- DOI: 10.3390/molecules21050615
Inhibitors of the Hydrolytic Enzyme Dimethylarginine Dimethylaminohydrolase (DDAH): Discovery, Synthesis and Development
Abstract
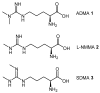
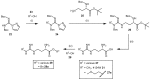
Dimethylarginine dimethylaminohydrolase (DDAH) is a highly conserved hydrolytic enzyme found in numerous species, including bacteria, rodents, and humans. In humans, the DDAH-1 isoform is known to metabolize endogenous asymmetric dimethylarginine (ADMA) and monomethyl arginine (l-NMMA), with ADMA proposed to be a putative marker of cardiovascular disease. Current literature reports identify the DDAH family of enzymes as a potential therapeutic target in the regulation of nitric oxide (NO) production, mediated via its biochemical interaction with the nitric oxide synthase (NOS) family of enzymes. Increased DDAH expression and NO production have been linked to multiple pathological conditions, specifically, cancer, neurodegenerative disorders, and septic shock. As such, the discovery, chemical synthesis, and development of DDAH inhibitors as potential drug candidates represent a growing field of interest. This review article summarizes the current knowledge on DDAH inhibition and the derived pharmacokinetic parameters of the main DDAH inhibitors reported in the literature. Furthermore, current methods of development and chemical synthetic pathways are discussed.
Keywords: arginine; asymmetric dimethylarginine; dimethylarginine dimethylaminohydrolase; enzyme inhibitors; monomethyl arginine; nitric oxide; organic synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
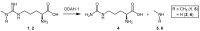




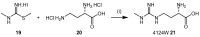









Similar articles
-
Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer.Front Oncol. 2020 Jan 9;9:1455. doi: 10.3389/fonc.2019.01455. eCollection 2019. Front Oncol. 2020. PMID: 31993367 Free PMC article. Review.
-
Arginine analogues incorporating carboxylate bioisosteric functions are micromolar inhibitors of human recombinant DDAH-1.Org Biomol Chem. 2015 Dec 14;13(46):11315-30. doi: 10.1039/c5ob01843a. Epub 2015 Sep 30. Org Biomol Chem. 2015. PMID: 26420019
-
Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase.Circ Res. 2003 Feb 7;92(2):226-33. doi: 10.1161/01.res.0000052990.68216.ef. Circ Res. 2003. PMID: 12574151
-
S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase.Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13527-32. doi: 10.1073/pnas.212269799. Epub 2002 Oct 7. Proc Natl Acad Sci U S A. 2002. PMID: 12370443 Free PMC article.
-
The DDAH/ADMA/NOS pathway.Atheroscler Suppl. 2003 Dec;4(4):33-40. doi: 10.1016/s1567-5688(03)00032-1. Atheroscler Suppl. 2003. PMID: 14664901 Review.
Cited by
-
Pharmacokinetic Characterization of the DDAH1 Inhibitors ZST316 and ZST152 in Mice Using a HPLC-MS/MS Method.Molecules. 2022 Feb 2;27(3):1017. doi: 10.3390/molecules27031017. Molecules. 2022. PMID: 35164277 Free PMC article.
-
Enhanced Nitric Oxide (NO) and Decreased ADMA Synthesis in Pediatric ADHD and Selective Potentiation of NO Synthesis by Methylphenidate.J Clin Med. 2020 Jan 8;9(1):175. doi: 10.3390/jcm9010175. J Clin Med. 2020. PMID: 31936392 Free PMC article.
-
Dissection, Optimization, and Structural Analysis of a Covalent Irreversible DDAH1 Inhibitor.Biochemistry. 2018 Jul 31;57(30):4574-4582. doi: 10.1021/acs.biochem.8b00554. Epub 2018 Jul 20. Biochemistry. 2018. PMID: 29983043 Free PMC article.
-
Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer.Front Oncol. 2020 Jan 9;9:1455. doi: 10.3389/fonc.2019.01455. eCollection 2019. Front Oncol. 2020. PMID: 31993367 Free PMC article. Review.
-
Analysis of Transcriptome Differences Between Subcutaneous and Intramuscular Adipose Tissue of Tibetan Pigs.Genes (Basel). 2025 Feb 20;16(3):246. doi: 10.3390/genes16030246. Genes (Basel). 2025. PMID: 40149398 Free PMC article.
References
-
- Greco R., Ferrigno A., Demartini C., Zanaboni A., Mangione A.S., Blandini F., Nappi G., Vairetti M., Tassorelli C. Evaluation of ADMA-DDAH-NOS axis in specific brain areas following nitroglycerin administration: Study in an animal model of migraine. J. Headache Pain. 2015;16, 74:1–8. doi: 10.1186/s10194-015-0560-2. - DOI - PMC - PubMed
-
- Hasegawa K., Wakino S., Kimoto M., Minakuchi H., Fujimura K., Hosoya K., Komatsu M., Kaneko Y., Kanda T., Tokuyama H., et al. The hydrolase DDAH2 enhances pancreatic insulin secretion by transcriptional regulation of secretagogin through a sirt1-dependent mechanism in mice. FASEB J. 2013;27:2301–2315. doi: 10.1096/fj.12-226092. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

